Anti-adsorption and long-circulation lipid molecule, preparation method and application thereof in fields of medicines and cosmetics
A lipid and molecular technology, applied in non-active ingredients medical preparations, pharmaceutical formulations, phosphorus organic compounds, etc., can solve problems such as difficulty in meeting multiple modifications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
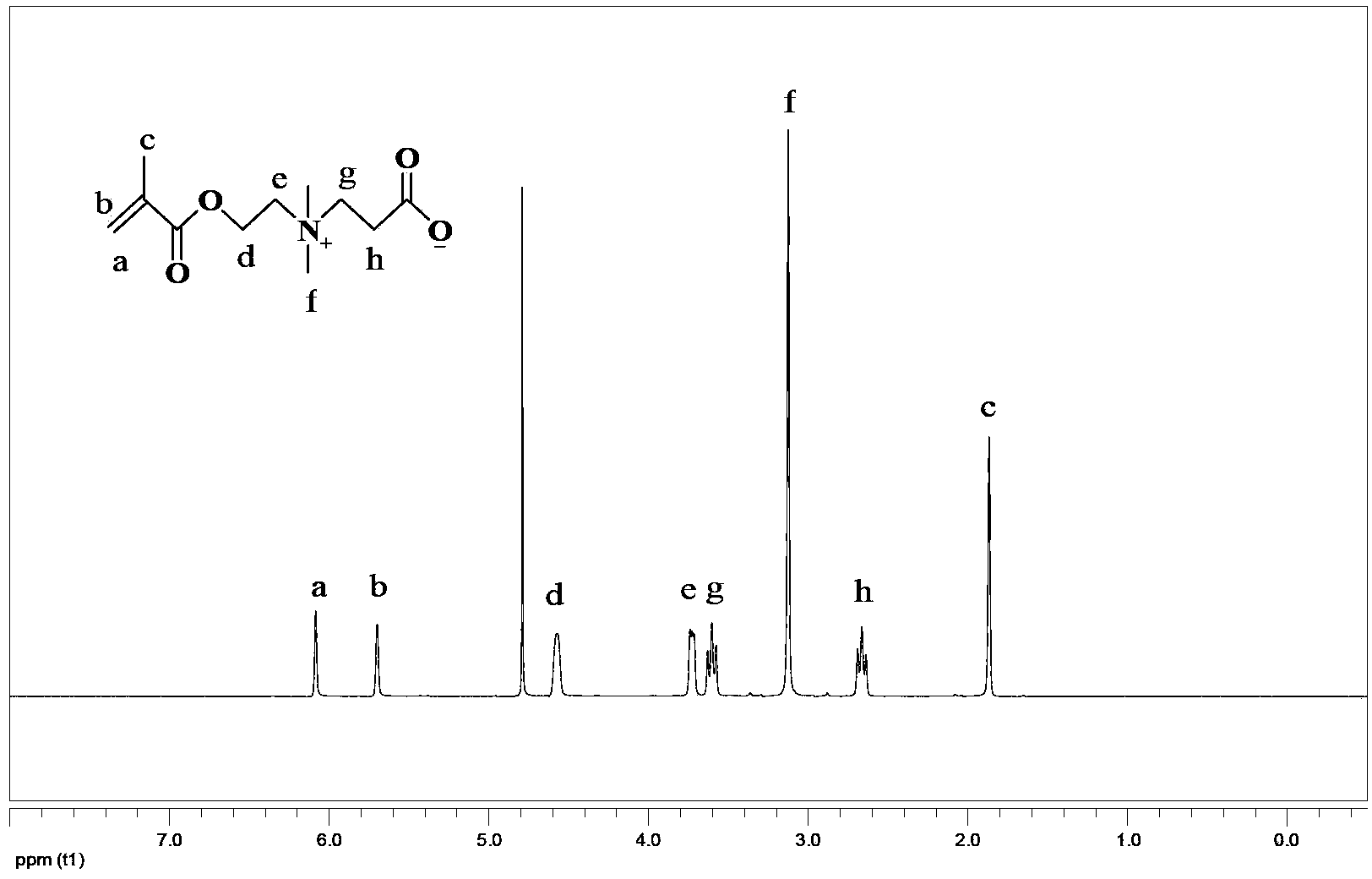
[0059] Synthesis of CBMA (compound (II))
[0060] Weigh 0.664g (3.97mmol) DMAEMA in a Schlenk bottle, and use N 2 Replace the oxygen in the bottle, in N 2 Under protection, add 20 mL of anhydrous dichloromethane with a disposable needle. After stirring at 25°C for about 10min, 0.343g (4.77mmol) of β-propiolactone was added rapidly, and stirred at 25°C for 5h. At the end of the reaction, use a rotary evaporator to remove the dichloromethane solvent, add an appropriate amount of acetone, and filter with suction to obtain a white solid, which is then washed with dichloromethane and ether, and dried in vacuo to obtain 0.8673 g of a white solid. figure 1 .
Embodiment 2
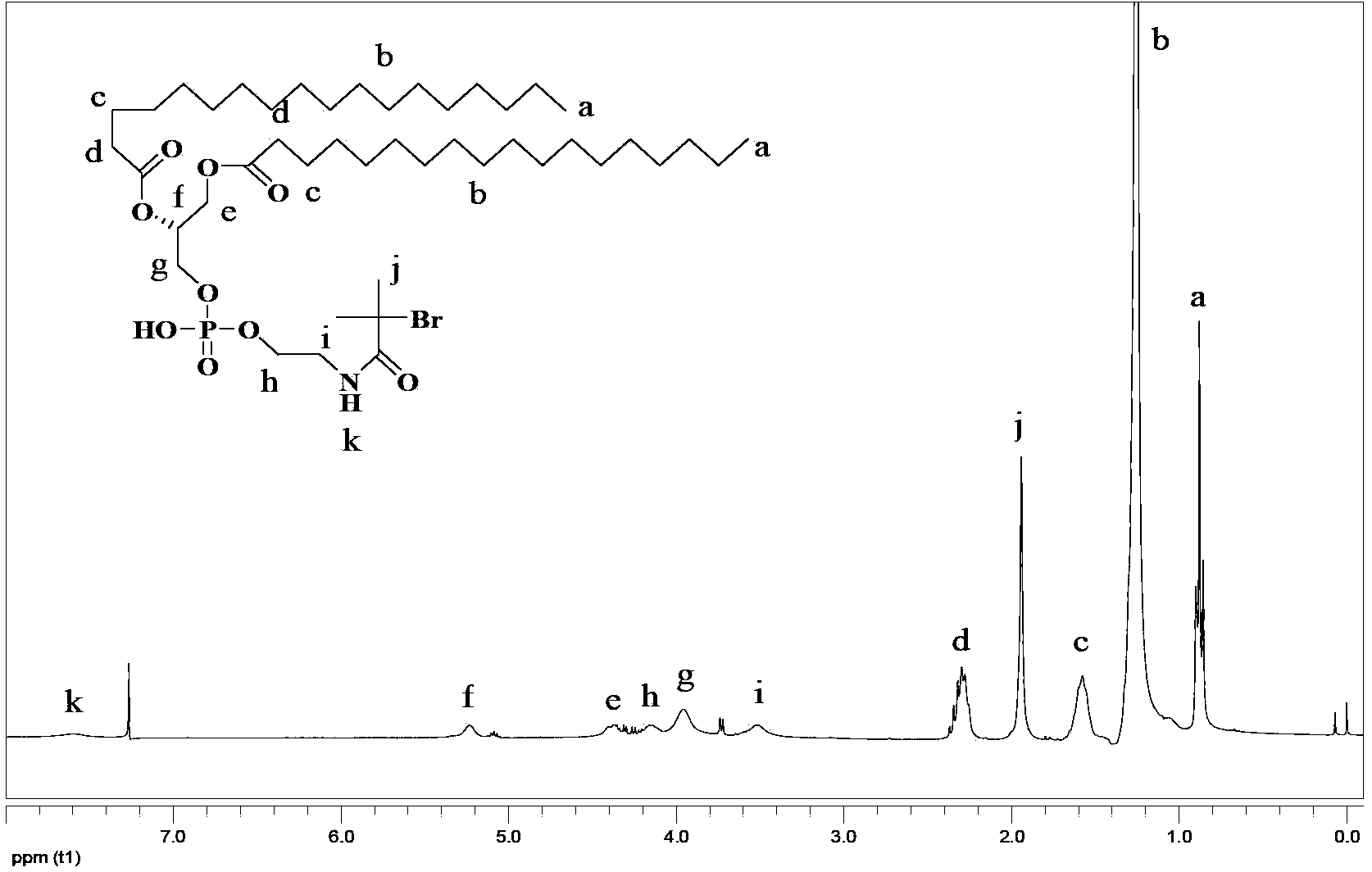
[0062] Synthesis of DSPE-Br (compound (III))
[0063] Take 0.2972g (0.40mmol) of DSPE in a flask, add 20mL of anhydrous dichloromethane and 0.0810g (0.80mmol) of triethylamine, and stir at 25°C for 1h. Add 0.1563g (0.68mmol) 2-bromo-2-methylpropionyl bromide to the above flask, heat to 45°C, condense and reflux for 12 hours. Transfer the reaction solution into a separatory funnel, add an equal volume of water to wash, and wash repeatedly 3 times. Add anhydrous sodium sulfate to the organic phase to dry, filter, and concentrate the filtrate to obtain 0.2674 g of a white solid, whose structural characterization is shown in the attached figure 2 .
Embodiment 3
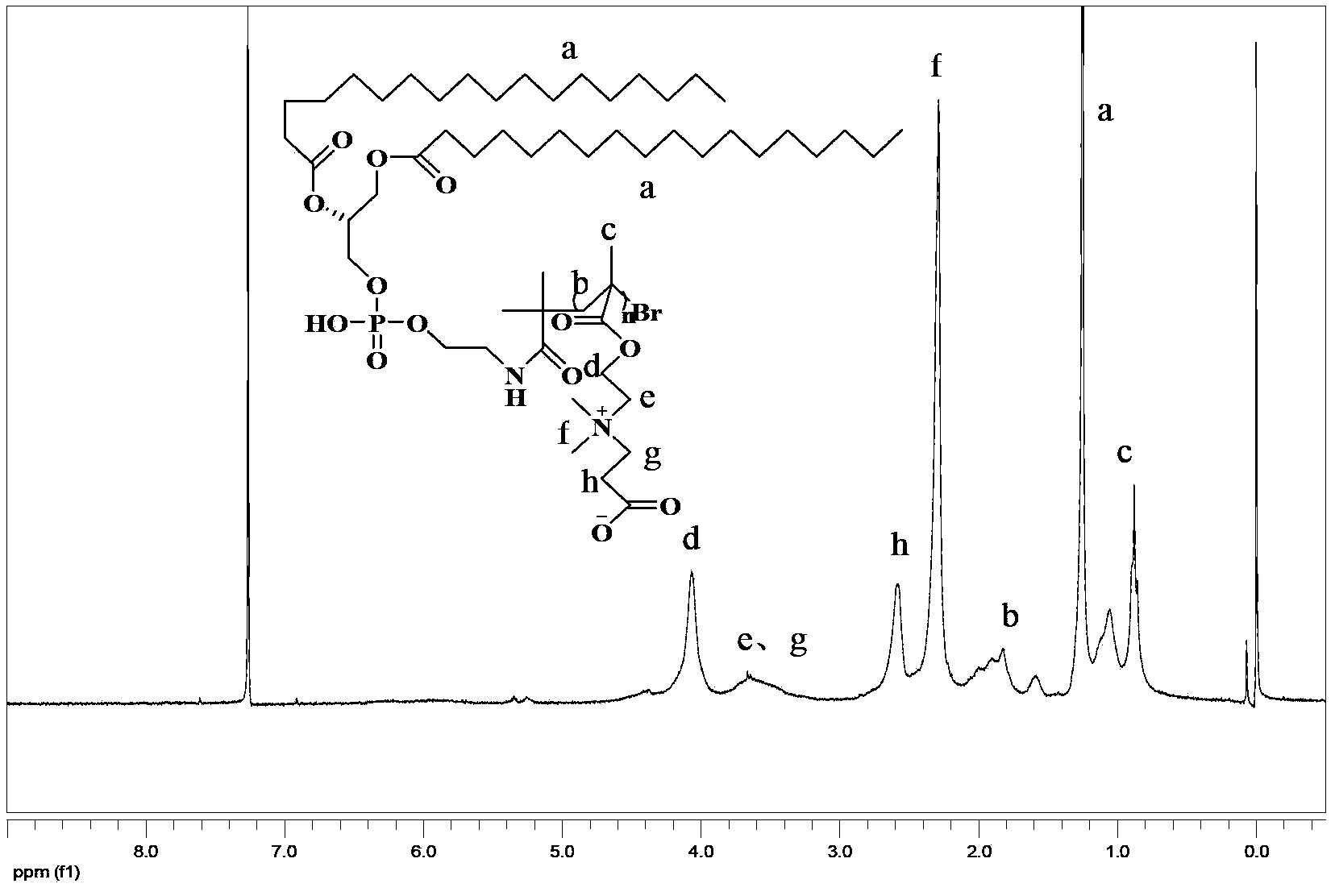
[0065] Synthesis of DSPE-PCBMA (compound I)
[0066]Taking the synthesis of PCBMA with a theoretical degree of polymerization of 50 as an example to illustrate the polymerization process of DSPE-PCBMA. Weigh 0.0358g (0.04mmol) of the DSPE-Br prepared in Example 2 and dissolve it in 2mL of dichloromethane, weigh 0.4586g (2.00mmol) of the CBMA prepared in Example 1 and dissolve it in 20mL of absolute ethanol, after mixing Add it to a clean dry Schlenk flask, add 12.0mg (0.08mmol) Cu(I)Br, and perform three freeze-thaw cycles to degas. Then measure 0.0139g (0.08mmol) PMDETA in 1mL of absolute ethanol, inject the solution into the frozen above reaction system, and then perform three freeze-thaw cycle degassing. The sealed reaction system was reacted at 60°C for 24 hours. The product was dialyzed in an osmotic bag with a molecular weight cut-off of 3500, respectively, using absolute ethanol and deionized water as the external phase, and freeze-dried to obtain 0.2173 g of a white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com