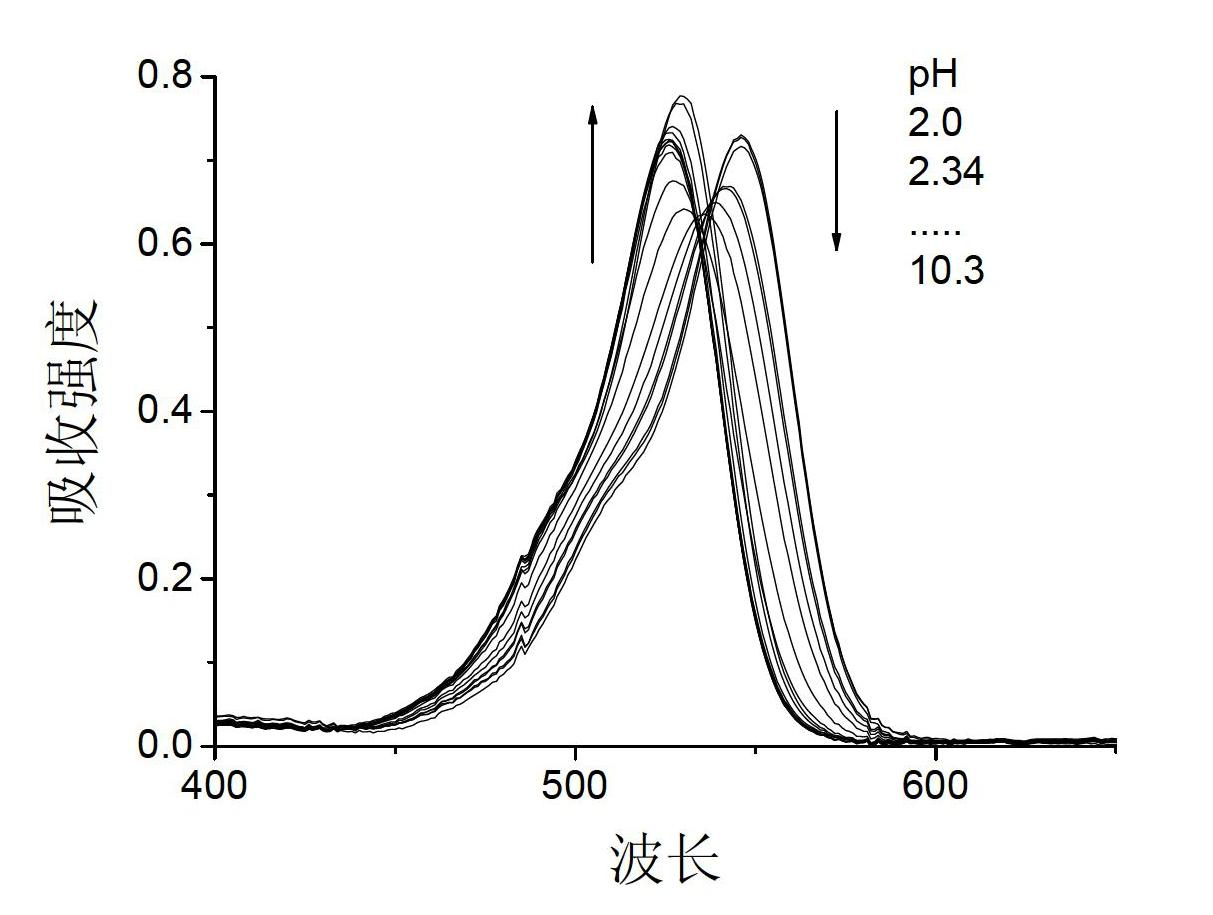
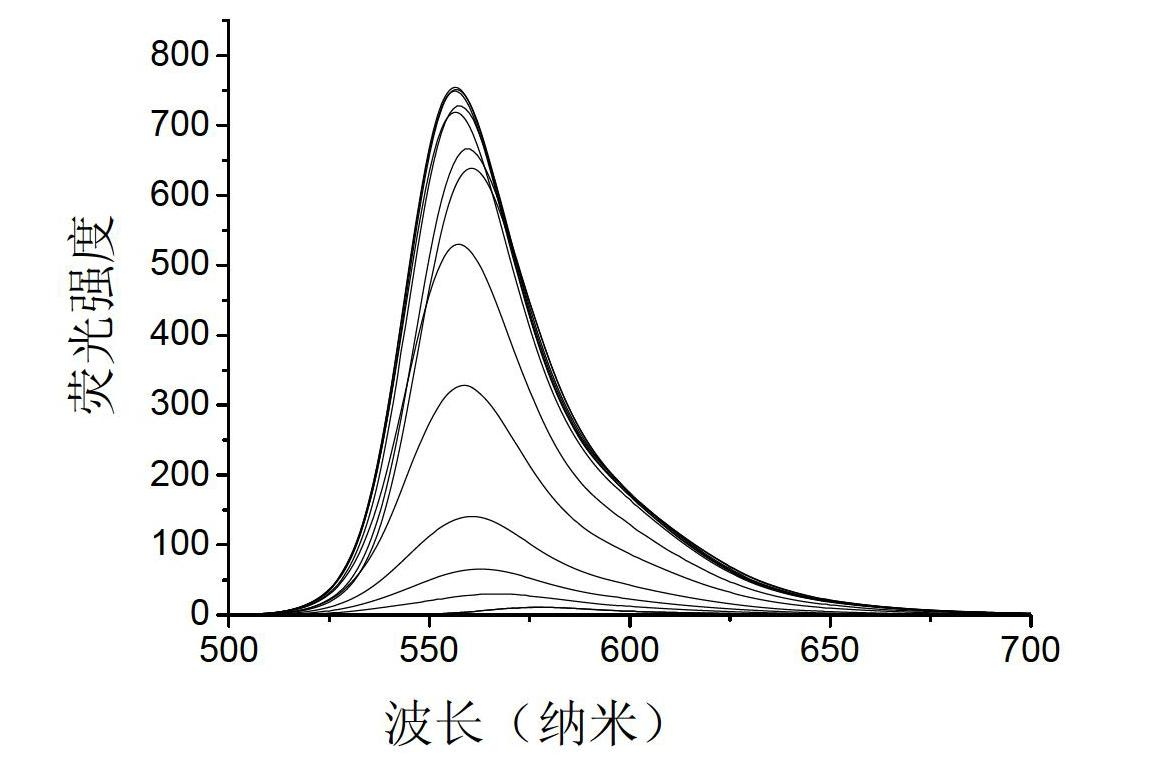
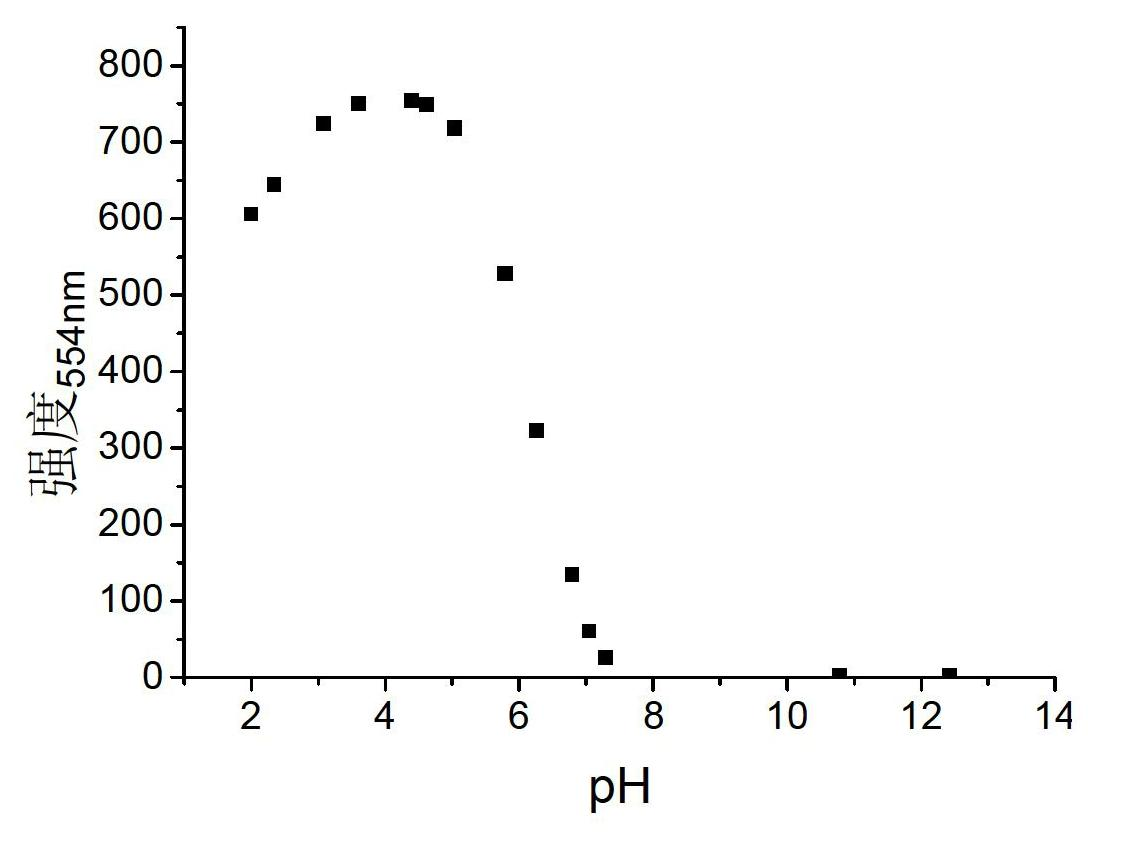
Rhodamine fluorescent probe sensitive to pH change, synthetic method and application
A fluorescent probe and synthesis method technology, applied in the field of rhodamine fluorescent probes, can solve problems such as changing the water solubility of rhodamine, unfavorable pH monitoring, etc., and achieve the effects of avoiding autofluorescence interference, reducing light damage, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] a. Dissolve dibromofluorescein in anhydrous toluene, add four times the amount of N-methylpiperazine, 20% palladium acetate, 20% BINAP, heat and reflux for 20 hours, evaporate the reaction solution to dryness under reduced pressure, and column Separate the target product, productive rate 60%, HRMS (M + ) 496.25
[0023] b. Dissolve the internal salt product obtained above in methanol, add 2 times the amount of concentrated hydrochloric acid dropwise and stir for 1 h, spin the methanol to dryness and purify to obtain a purple solid.
example 2
[0025]
[0026] c. Dissolve the product Example 1 in methanol, dropwise add 2 times the amount of thionyl chloride to raise the temperature and reflux for reaction, cool down and spin the methanol to dry and purify to obtain a purple powder solid. Yield 90%, HRMS(M + ) 511.2703
example 3
[0028]
[0029] Replace methyl alcohol with ethanol in c operation, all the other are with example 2. Yield 90%, HRMS (M + )525.2860
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com