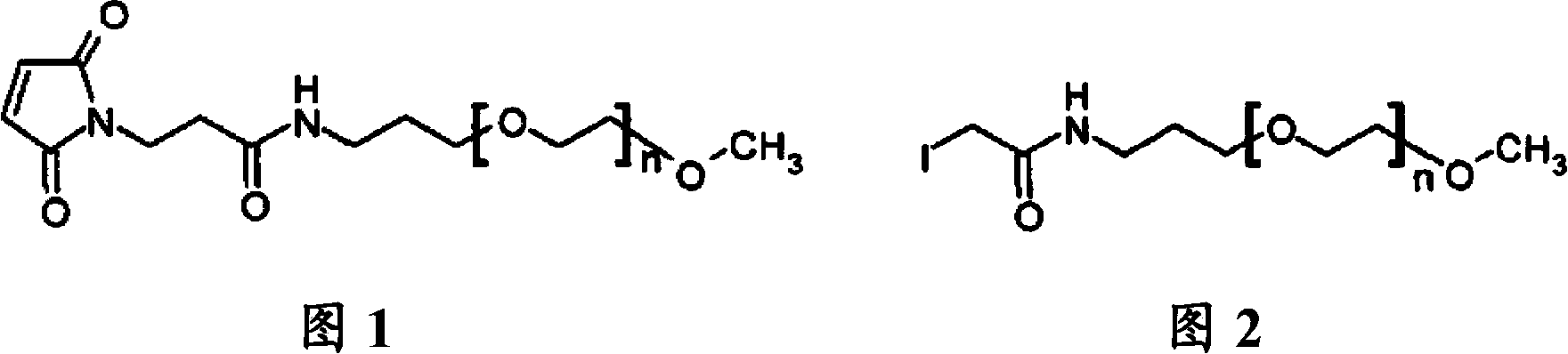Oxyntomodulin peptide analogue
A technology of oxyntomodulatory peptides and analogues, applied in the direction of hormone peptides, specific peptides, drug combinations, etc., to achieve good weight and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Peptide Synthesis
[0096] Peptide analogues of SEQ ID NO: 1 and SEQ ID NO: 2 according to the present invention were generated by solid peptide synthesis on a Protein Technologies Inc. Symphony or Applied Biosystems 433A automated peptide synthesizer. Synthesis was performed on Fmoc-Rink amide polystyrene resin (Rapp Polymere Tubingen, Germany) with a substitution rate of about 0.7 mmol / g. Synthesis was performed using the Fmoc backbone protecting group strategy. The amino acid side chain derivatives used are: Arg(Pbf), Asn(Trt), Asp(OtBu), Cys(Trt), Gln(Trt), Glu(OtBu), His(Trt), Lys(Boc), Ser (OtBu), Thr(OtBu), Trp(Boc) and Tyr(OtBu). Amino acids activated by diisopropylcarbodiimide (DIC) and hydroxybenzotriazole (HOBt) in dimethylformamide (DMF) or N-methylpyrrolidone (NMP) (1:1:1 molar ratio) about 10 equivalents to perform the coupling. Couplings were performed at room temperature for 45-90 minutes.
[0097]Containing trifluoroacetic acid (TFA): tr...
Embodiment 2
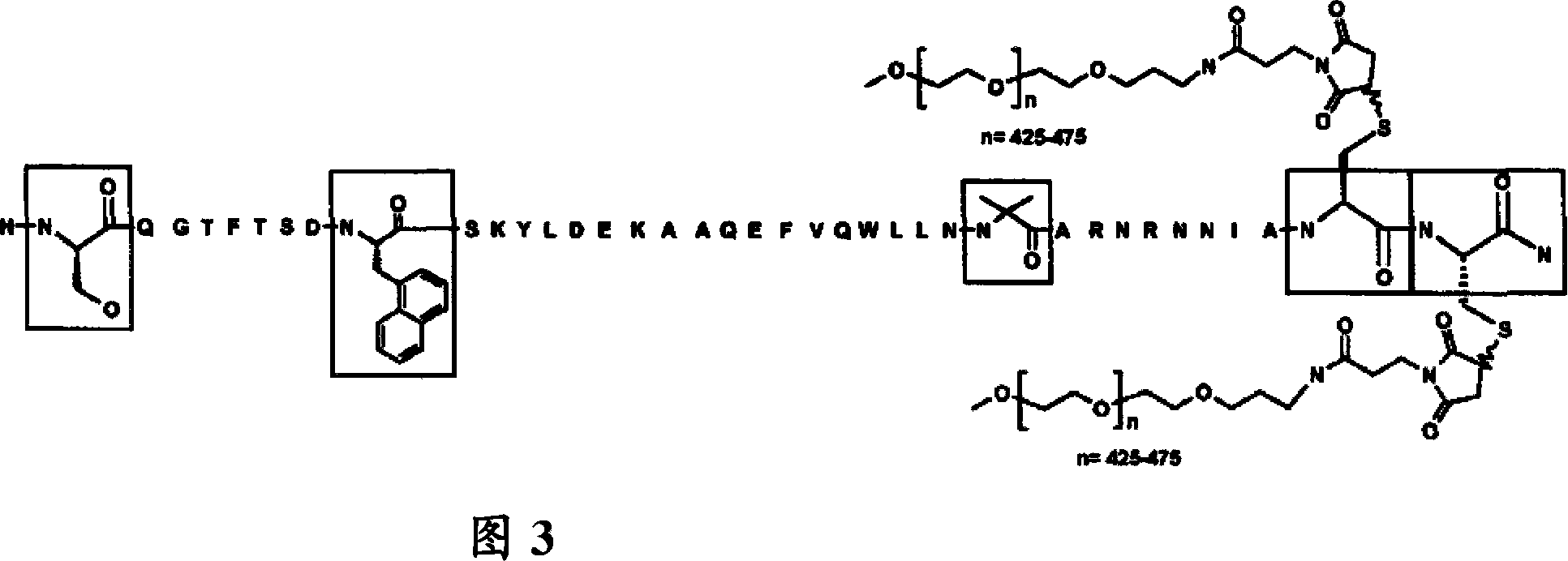
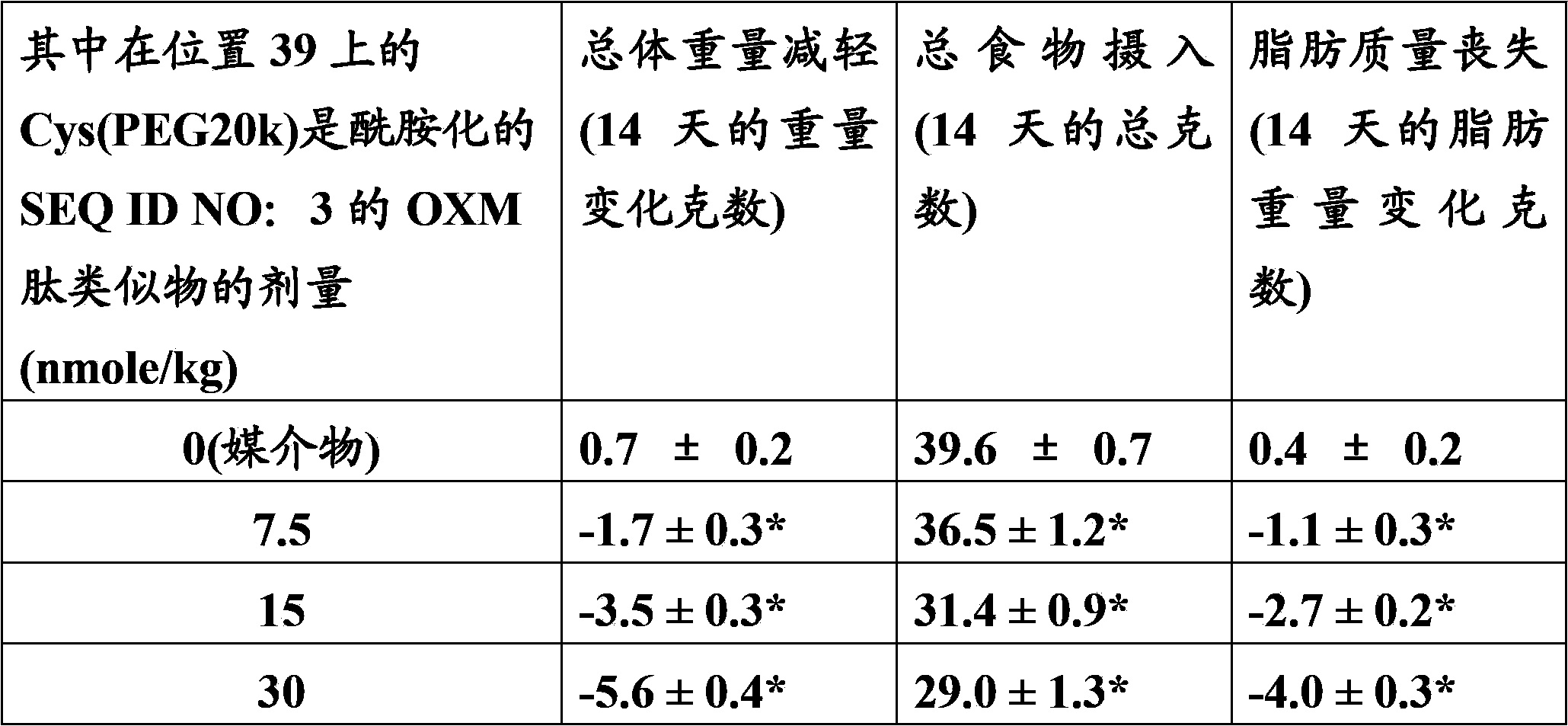
[0098] Example 2: Pegylation of a peptide containing 2 Cys residues by mPEG-MAL-20kDa
[0099] The lyophilized peptide analog (SEQ ID NO: 2) produced according to Example 1 was weighed out (typically 30-50 mg). 2.1 molar equivalents of mPEG-20 kDa maleimide (CH3O(CH2CH2O)n-(CH2)3NHCO(CH2)2-maleimide) (NOF Sunbright ME-200MA) were weighed out and combined with the peptide. The reaction was dissolved in a 50 / 50 (v / v) water / acetonitrile mixture to a peptide concentration of approximately 20 mg / mL. The peptide analog solution was diluted 2-fold with 100 mM ammonium acetate, 10 mM ethylenediaminetetraacetic acid (EDTA), pH 7. The resulting mixture was then stirred at room temperature. The reaction mixture was monitored by analytical reverse-phase HPLC (analytical HPLC separation was performed on a Waters SymmetryShield C18, 3.5 micron, 4.6 mm i.d. x 10 cm column at 50° C. using a 2-stage linear AB gradient—5 minutes 0-30% B and Followed by 30 min 30-90% B, where A = 0.05% TFA / HO...
Embodiment 3
[0100] Example 3: Glucagon Receptor (hGcgR) Binding Assay
[0101] Glucagon receptor binding assay utilizing cloned human glucagon receptor (hGcgR) isolated from 293HEK membrane (Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, Mathewes S, Grant FJ, Biggs SH, Rosenberg GB, et al. Gene 140(2), 203-209 (1994)). The hGlucR cDNA was subcloned into expression plasmid phD (Trans-activated expression of fully gamma-carboxylated recombinant human protein C, an antithrombotic factor. Grinnell, B.W., Berg, D.T., Walls, J. and Yan, S.B. Bio / Technology 5: 1189-1192 (1987)). This plasmid DNA was transfected into 293HEK cells and selected with 200 μg / ml hygromycin.
[0102] Crude plasma membranes were prepared using cells from suspension culture. Cells were lysed on ice in hypotonic buffer containing 25 mM Tris HCl, pH 7.5, 1 mM MgCl2, DNAse1, 20 ug / ml, and Roche complete inhibitor without EDTA. Homogenize the cell suspension with 25 strokes using a Teflon pestle with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com