Guanidyl modified quaternary ammonium salt and synthetic method thereof
A synthesis method and technology of quaternary ammonium salts are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of strong positive electricity, high antibacterial activity and significant bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
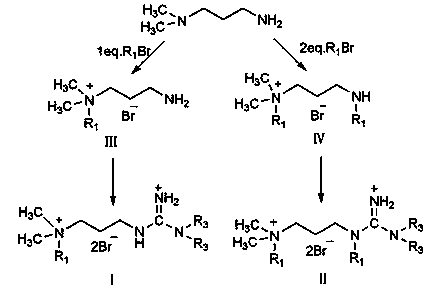
[0030] Embodiment 1: the synthesis of N,N-dimethyl-N-guanidine propyl n-octyl quaternary ammonium salt
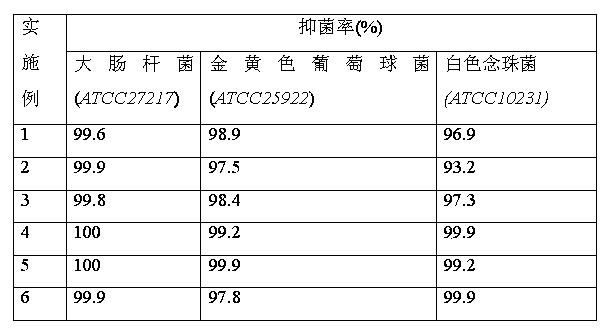
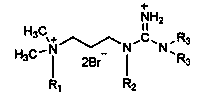
[0031] Add 5.1g (0.05mol) of N,N-dimethylpropylenediamine and 50g of absolute ethanol into a 250ml four-necked flask, and slowly add 9.7g (0.05mol) of n-bromooctane dropwise under stirring at room temperature. ), after 30min dropwise addition, continue to stir for 1h, then heat up and reflux for 4h, distill off ethanol under reduced pressure, wash with ether twice, add acetone 30g, hydrobromic acid (content 10%) to pH = 4, drop Add 5.1 g of cyanamide solution (50% aqueous solution), reflux for 2 hours, remove acetone under reduced pressure, and recrystallize to obtain the product N,N-dimethyl-N-guanidinepropyl n-octyl quaternary ammonium salt with a yield of 74%.
[0032] Infrared spectrum ( KBr, cm -1 ): 3366, 3280, 2936, 2857, 1646(C=N, st) (C=N, st) (C=N, st) (C=N, st), 1406(N-H, δ), 1114(C –N–C, δ), 721.
[0033] Proton NMR spectrum (400MHz, D 2 O), δ / ppm, 0.82(t, 3...
Embodiment 2
[0036] Embodiment 2: the synthesis of N,N-dimethyl-N-(dimethylguanidine-propyl) n-decyl quaternary ammonium salt
[0037] Add 5.1g (0.05mol) of N,N-dimethylpropylenediamine and 30g of n-propanol into a 250ml four-necked flask, and slowly add 11.1g of n-bromodecane dropwise under stirring at room temperature for 20min. After the addition is complete, continue to stir and react for 0.5 h, raise the temperature and reflux for 6 h, distill off the solvent under reduced pressure, wash with diethyl ether twice, then adjust the pH to 3 with 50 g of acetone and hydrobromic acid (content 30%), add 5.3 g of N, N -Dimethylcyanamide, reflux for 5h, remove the solvent under reduced pressure, and recrystallize to obtain the product methyl-substituted guanidinyl dimethyl n-decyl quaternary ammonium salt, with a yield of 82%.
[0038] Infrared spectrum ( KBr, cm -1 ): 3419, 2925, 2856, 1646, 1406, 1114, 721.
[0039] Proton NMR spectrum (400MHz, D 2 O), δ / ppm, 0.85(t, 3H, CH3 ), 1.27 (br...
Embodiment 3
[0042] Embodiment 3: the synthesis of N,N-dimethyl-N-guanidine propyl dodecyl quaternary ammonium salt
[0043] Add 5.1g (0.05mol) of N,N-dimethylpropylenediamine and 30g of isopropanol into a 250ml four-necked flask, and slowly add 12.5g of bromododecane dropwise under stirring at room temperature for 80 min After the dropwise addition is completed, continue to stir for 2 hours, then heat up to reflux for 8 hours, distill off the solvent under reduced pressure, wash with ether twice, add acetone, adjust the pH to 2 with hydrobromic acid (content 10%), add 6.3 g of cyanamide solution dropwise (50% aqueous solution), reflux for 6 hours, remove the solvent under reduced pressure, and recrystallize from ethanol to obtain the product N,N-dimethyl-N-guanidinepropyl dodecyl quaternary ammonium salt with a yield of 87%.
[0044] Infrared spectrum ( KBr, cm -1 ): 3366, 3280, 2925, 2856, 1646, 1406, 1114, 721.
[0045] Proton NMR spectrum (400MHz, D 2 O), δ / ppm, 0.84(t, 3H, CH3), 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com