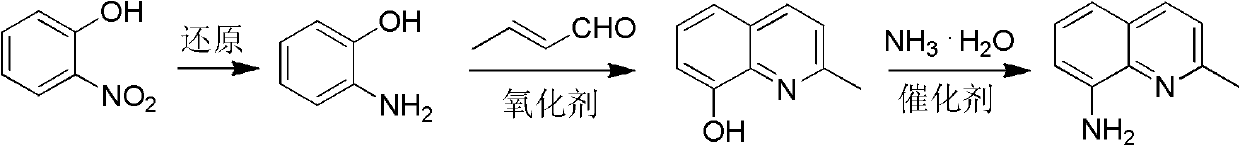
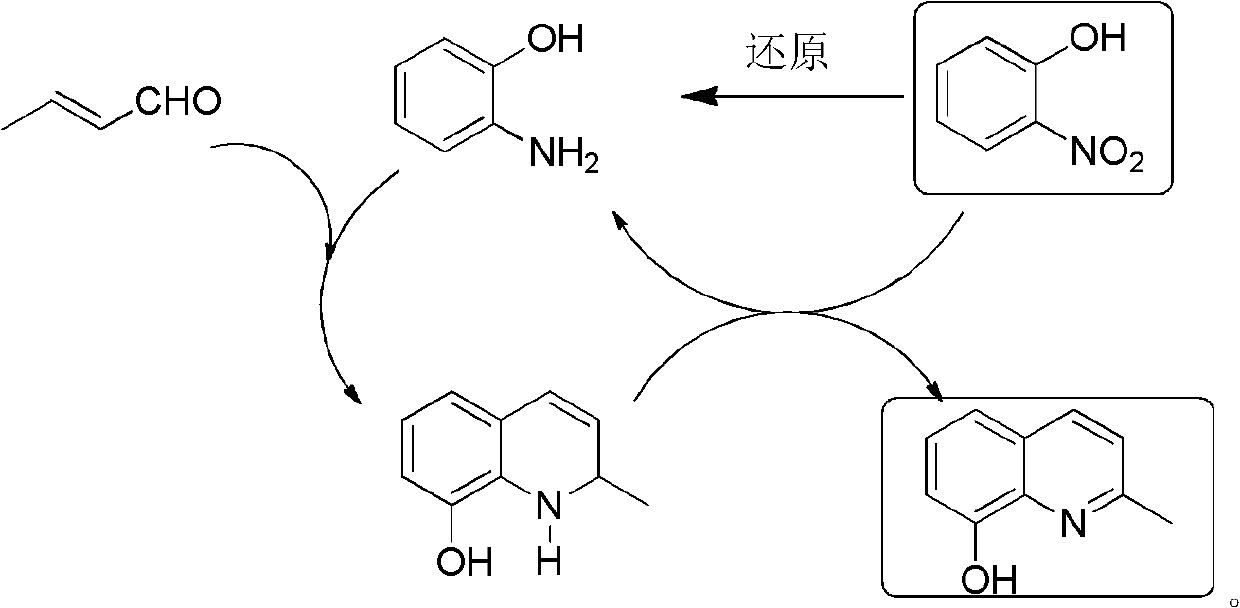
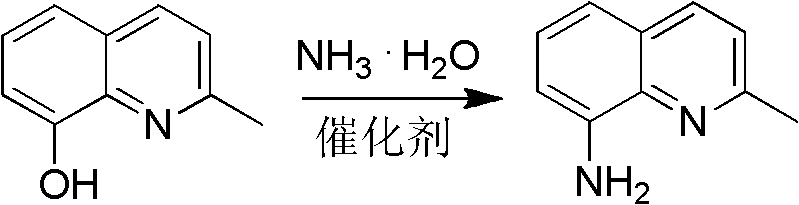
Method for preparing 2-methyl-8-aminoquinoline from o-nitrophenol
A technology of aminoquinoline and o-nitrophenol, applied in the direction of organic chemistry, can solve the problem of difficult preparation of 2-methyl-8-aminoquinoline, difficult amino substitution reaction, separation and purification of 2-methyl-8- Solve the problems of nitroquinoline, etc., and achieve the effect of novel preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of o-aminophenol
[0038]
[0039] O-nitrophenol was catalytically hydrogenated (Pd / C, H 2 ) or chemical reduction (Na 2 S) make o-aminophenol.
Embodiment 2
[0041] Preparation of 2-methyl-8-hydroxyquinoline
[0042]
[0043] 33.0g (0.3mol) o-aminophenol 150mL 18% HCl stirred and refluxed; 0.5h dropwise added 14.0g (0.1mol) o-nitrophenol and 42.0mL (0.4mol) crotonaldehyde solution, refluxed for 2h, cooled, neutralized with ammonia water, Extract with toluene (100mL×4), dry over anhydrous sodium sulfate, and rotate the solvent to obtain a black solid, distill under reduced pressure, collect fractions at 149~155℃ / 246Pa to obtain 2-methyl-8-hydroxyquinoline, mp 71~ 72°C, yield ≥ 85%. MS (m / z): 159 (M + ). 1 H NMR (CDCl 3 , 400MHz) δ: 2.71 (s, 3H, CH 3 ), 7.13(d, J=7.2Hz, 1H, quinoline ring 7-H), 7.22~7.30(m, 2H, quinoline ring 3, 6-H), 7.32~7.41(m, 1H, quinoline ring 5-H), 8.00 (d, J = 8.4 Hz, 1H, quinoline ring 4-H).
Embodiment 3
[0045] Preparation of 2-methyl-8-aminoquinoline
[0046]
[0047] 2-methyl-8-hydroxyquinoline 31.8g (0.20mol), ammonium chloride (0.10mol), cobalt chloride (0.02mol), 22-28% ammonia water 120mL, filled with N 2 Gas, heated to 300-320°C, pressure 1.8-2.2MPa, pressure-holding and heat-retaining reaction for 4-6h; after the reaction, the reaction solution was extracted with toluene, the organic layer was collected, dried, and the solvent was recovered to obtain a dark gray solid, which was distilled under reduced pressure 2-methyl-8-aminoquinoline is obtained with a yield of ≥70%. mp: 56-58°C. 1 H NMR (CDCl 3 , 400MHz) δ: 2.70 (s, 3H, CH 3 ), 4.97 (brs, 2H, NH 2 ), 6.89 (d, J=7.2Hz, 1H, quinoline ring), 7.10 (d, J=8.0, 1H, quinoline ring), 7.24 (m, 2H, quinoline ring), 7.93 (d, J= 8.0, 1H, quinoline ring).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com