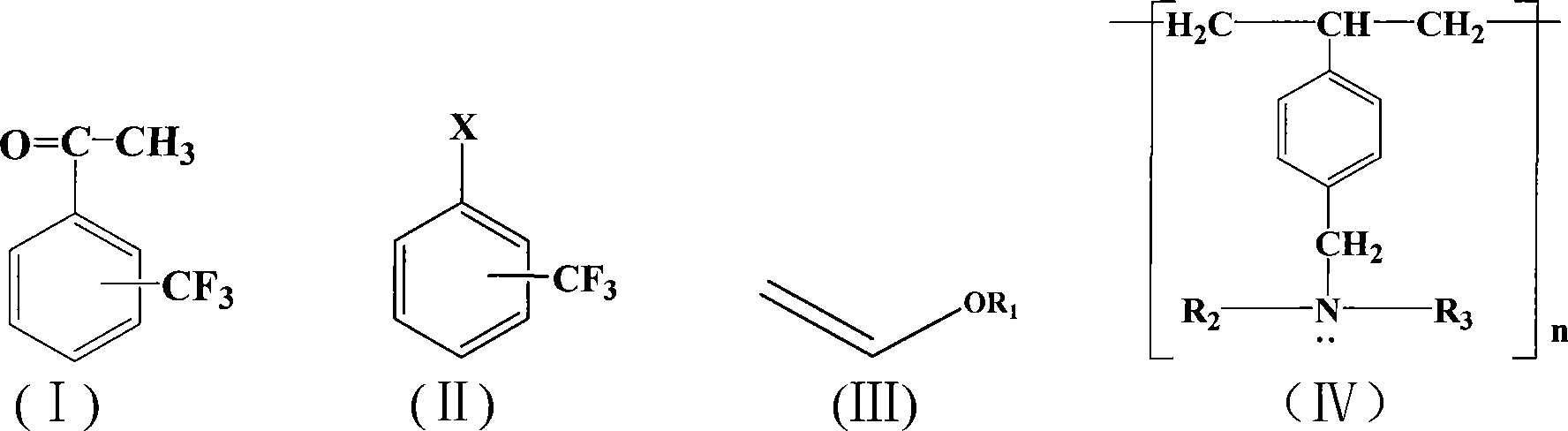
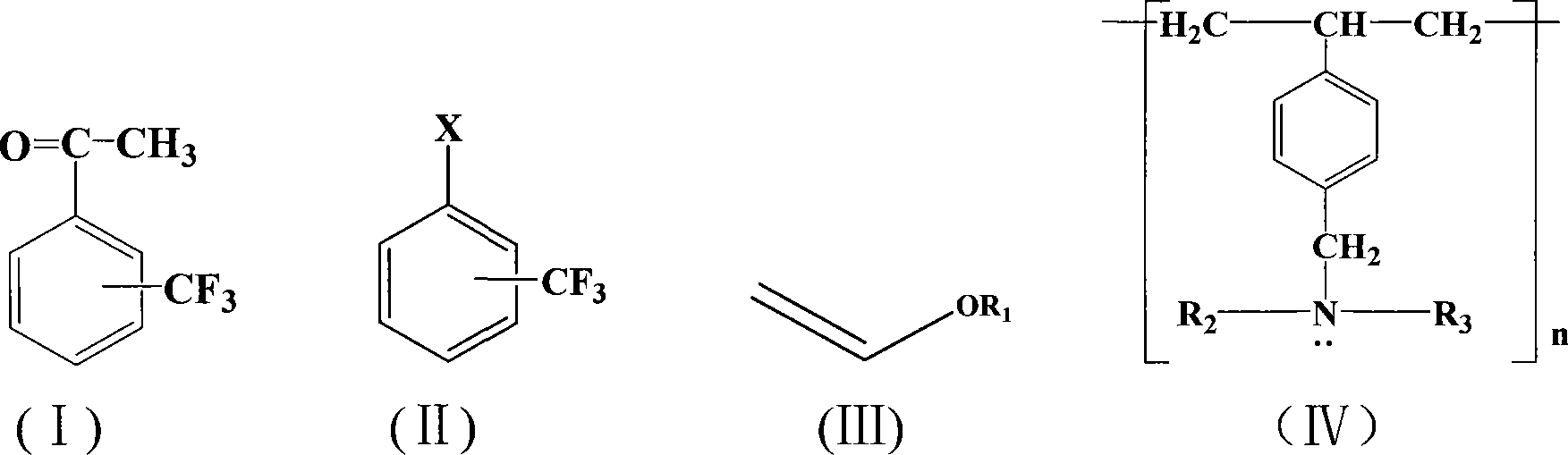
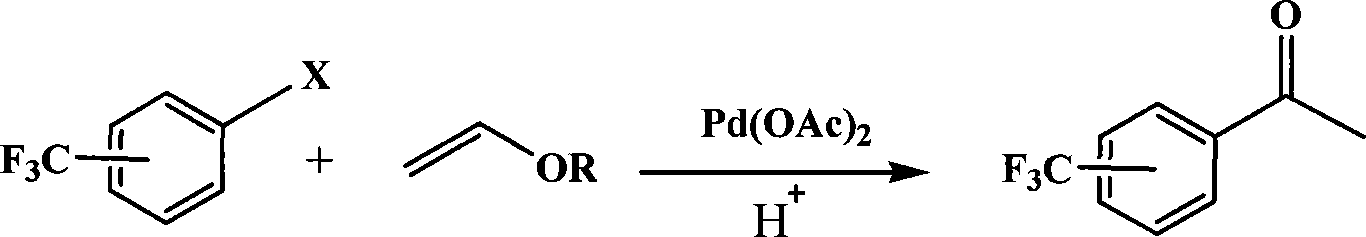A method for preparing trifluoromethyl acetophenone compound
A technology of trifluoromethyl acetophenone and trifluoromethyl halogenated benzene, which is applied in the field of preparation of trifluoromethyl acetophenone compounds, can solve the problems of expensive starting materials, low yield, easy diazomethane Explosion and other problems, to achieve the effect of novel preparation process, high product purity, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of m-trifluoromethyl acetophenone
[0021] 0.18 g (1 mmol) of m-trifluoromethyl chlorobenzene, 0.5 g (5 mmol) of vinyl butyl ether, 0.0012 g (0.005 mmol) of palladium acetate, 0.3 g (1.5 mmol) of Styrene-DVB (D301 R) resin millimoles), 10 ml of dimethyl sulfoxide, placed in a 200 ml three-necked flask, stirred and heated, and reacted at 100° C. for 1 hour. After the reaction, cool, add 10% dilute hydrochloric acid to acidify to pH=3, stir for 30 minutes, extract with toluene (3×50 ml) (extract 3 times, 50 ml each time, the same below), dry the extract, and concentrate , 0.095 g of the product was separated by column chromatography, and the yield was 50%. Boiling point 198~202℃, purity ≥98%, MS(m / z): 188(M + ).
Embodiment 2
[0022] Embodiment 2: the preparation of m-trifluoromethyl acetophenone
[0023] 0.22 g (1 mmol) of m-trifluoromethyl bromide, 0.2 g (2 mmol) of vinyl butyl ether, 0.0012 g (0.005 mmol) of palladium acetate, 0.4 g (2 mmol) of Styrene-DVB (D301 R) resin millimoles), 100 milliliters of dimethylformamide were placed in a 200 milliliter three-necked flask, stirred and heated, and reacted at 150° C. for 10 hours. After the reaction, cool, add 10% dilute hydrochloric acid to acidify to pH=3, stir for 30 minutes, extract with toluene (3×50 ml), dry the extract, concentrate, and separate by column chromatography to obtain 0.13 g of the product, with a yield of 70 %. Boiling point 198~202℃, purity ≥98%, MS(m / z): 188(M + ).
Embodiment 3
[0024] Embodiment 3: the preparation of p-trifluoromethylacetophenone
[0025] 0.22 g (1 mmol) of p-trifluoromethylbromobenzene, 0.2 g (2 mmol) of vinyl butyl ether, 0.0012 g (0.005 mmol) of palladium acetate, 0.3 g (1.5 mmol) of Styrene-DVB (D301 R) resin millimoles), 50 milliliters of 1-methylpyrrolidone were placed in a 200 milliliter three-necked flask, stirred and heated, and reacted at 50° C. for 50 hours. After the reaction, cool, add 10% dilute hydrochloric acid to acidify to pH=3, stir for 30 minutes, extract with toluene (3×50 ml), dry the extract, concentrate, and separate by column chromatography to obtain 0.155 g of the product, with a yield of 80 %. Melting point 30~32℃, purity ≥98%, MS(m / z): 188(M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com