Branched polyacrylamide and preparation method thereof
A polyacrylamide and acrylamide technology, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and preparation of organic compounds, etc., can solve the problem of the decrease in the ability of polymers to establish flow resistance, the loss of polymer solution viscosity, and the decrease in solution viscosity, etc. problems, to achieve the effects of excellent mechanical shear resistance, easy industrial production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
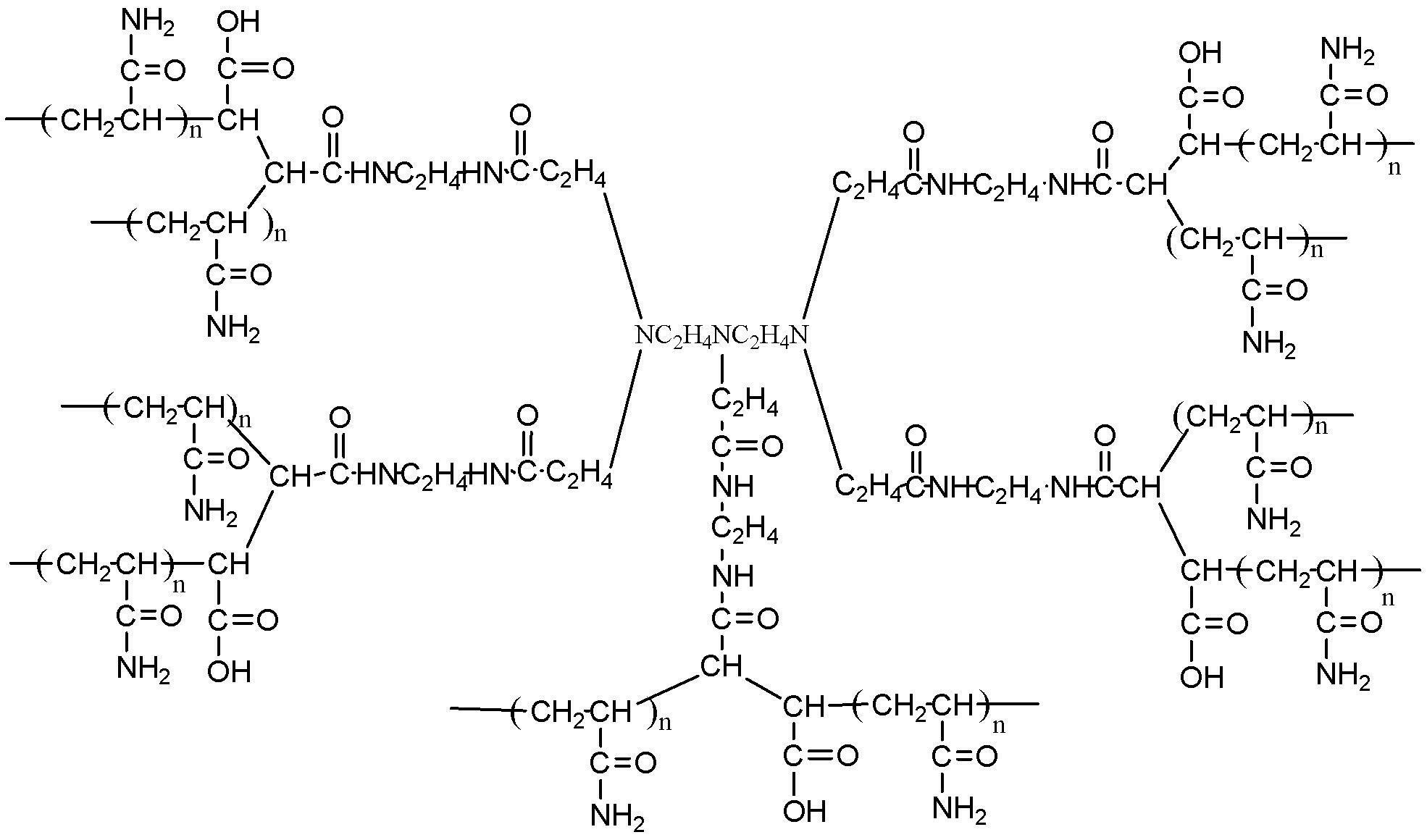
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of branched polyacrylamide
[0035] (1) Synthesis of branched functional monomer
[0036] Put a 1000ml single-necked round-bottomed flask into an oil bath, add 0.1 mol of diethylene triamine and 0.5 mol of methyl acrylate, dimethyl sulfoxide as a solvent, and react for 6 hours under uniform stirring in an ice bath, and then add 1.0 mol The ethylenediamine undergoes transesterification reaction at 80°C. After 6 hours, the dimethyl sulfoxide and excess unreacted ethylenediamine are all distilled out under reduced pressure with a diaphragm vacuum pump for 4 hours. Then 0.5 mol of maleic anhydride in dimethyl sulfoxide was added to the flask, heated to 80°C for 6 hours, and then distilled under reduced pressure to remove dimethyl sulfoxide to obtain a white branched functional monomer sample.
[0037] (2) Synthesis of branched polyacrylamide
[0038] 1000g of acrylamide, 0.05g of branched functional monomer, dissolved in 4000g of distilled water, protected by n...
Embodiment 2
[0039] Example 2 Structural characterization of branched functional monomers
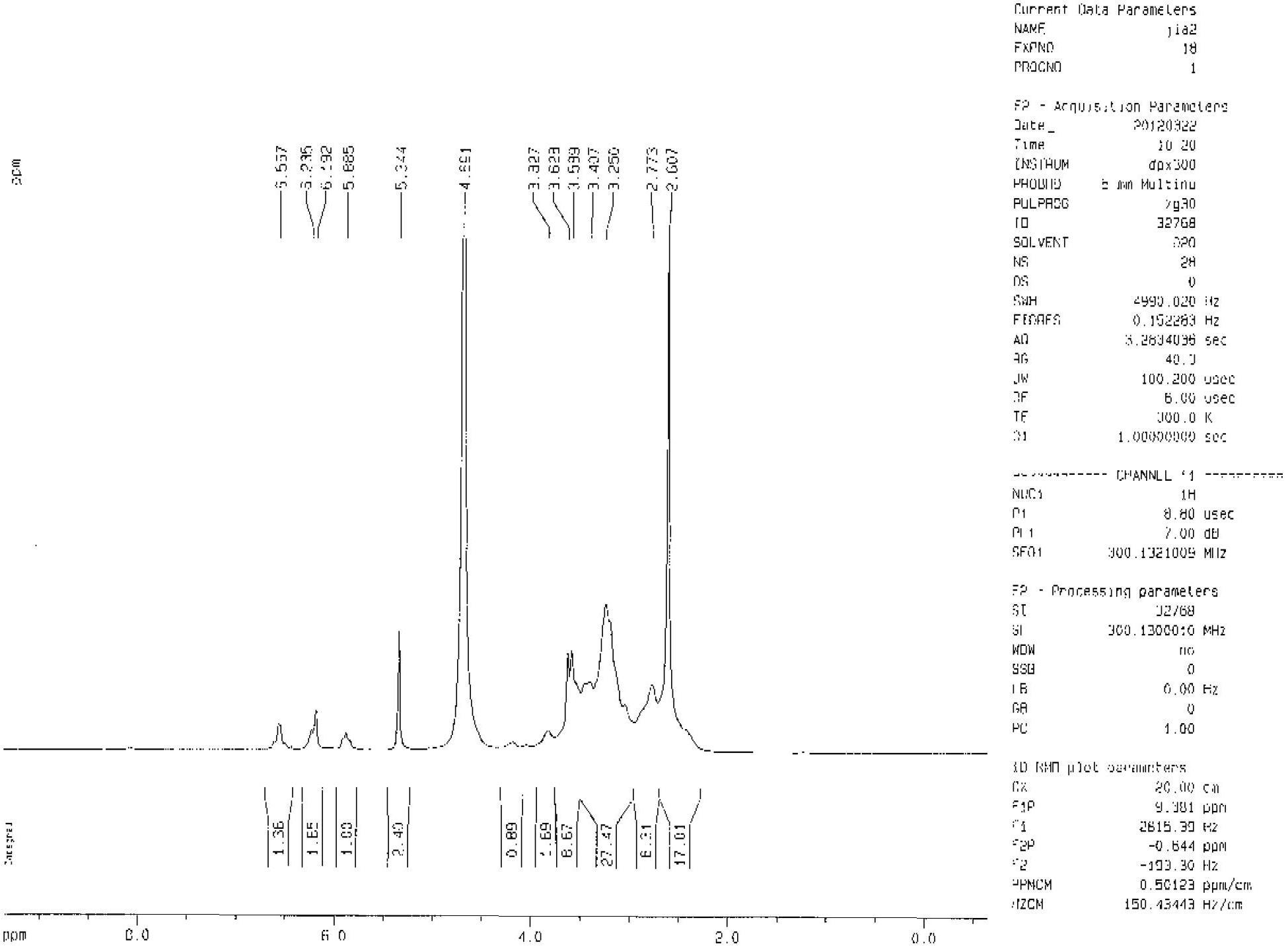
[0040] figure 1 It is the proton nuclear magnetic resonance spectrum of the synthesized branched functional monomer. The functional monomer uses D 2 O is dissolved and TMS is used as an internal standard. In the target molecule, each hydrogen atom corresponds to the following:
[0041] The proton shift δ is: methyl group (3.250), methylene group adjacent to amide group (around 3.407 and 3.628), methylene group adjacent to tertiary amine (2.773), CH 2 =CH double bond (near 5.885 and 6.567).
[0042] From the analysis of the spectrum data, it is known that the product is a branched functional monomer.
Embodiment 3
[0043] Example 3 Characterization of the structure of branched polyacrylamide
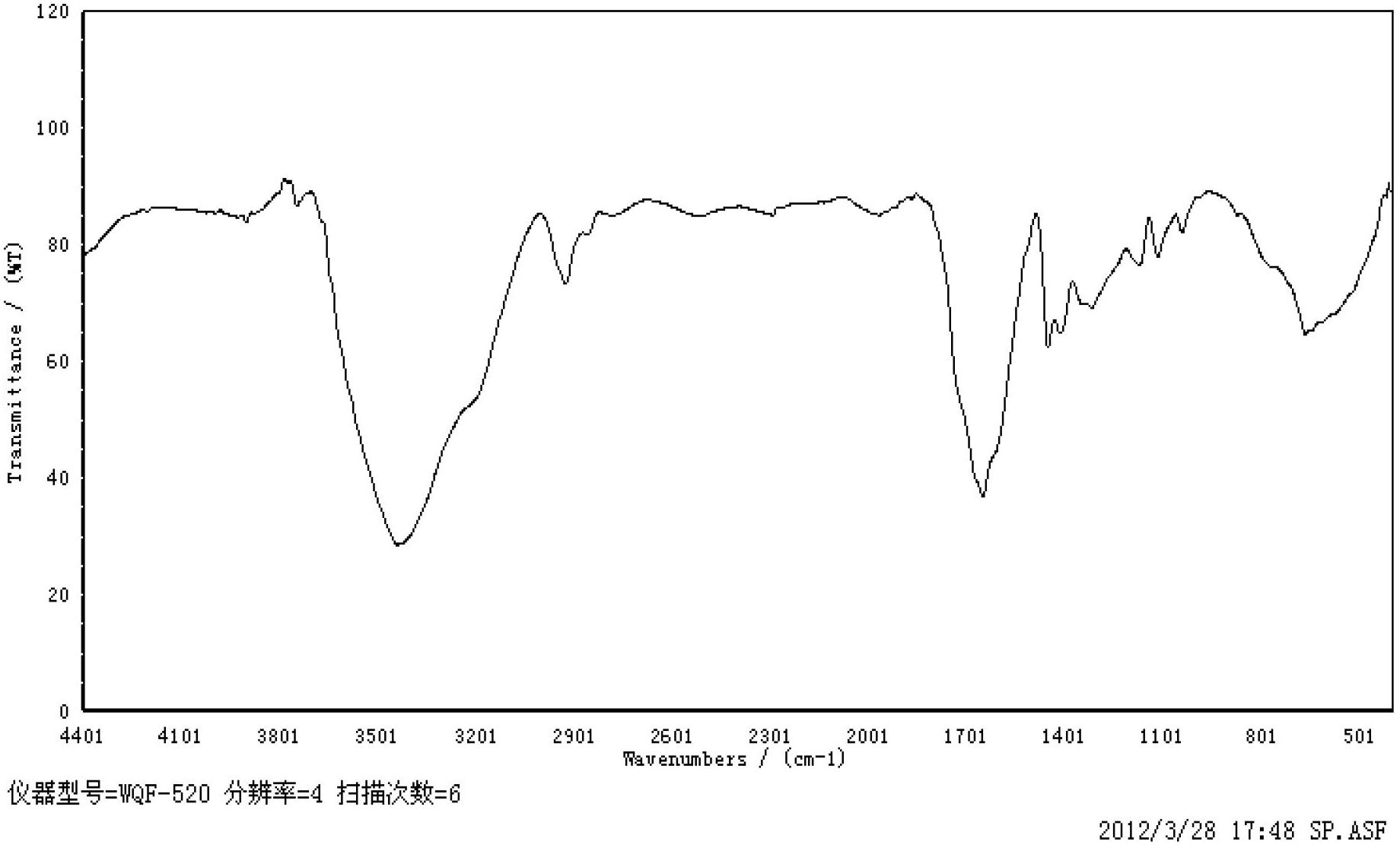
[0044] figure 2 It is the infrared spectrum of branched polyacrylamide, which can be analyzed from the spectrum: 3430 cm -1 Is the hydroxyl stretching vibration peak, 2920 cm -1 Stretching vibration peak of methylene group, 1650cm -1 Is the amide and secondary amide stretching vibration peak; 1450 cm -1 There is the peak of hydrocarbon bending vibration; 1350 cm -1 It is the absorption peak of bending vibration produced by hydroxyl group.
[0045] Infrared spectroscopy analysis of various functional groups of the polymer showed that the synthesized polymer has the characteristics of branched polyacrylamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com