Electron withdrawing end group modified fluorene-pyrene derivative blue-ray material
A technology of pyrene derivatives and blue light materials, which is applied in the direction of luminescent materials, organic chemistry, halogenated hydrocarbon preparation, etc., can solve the problem of difficult improvement of the chemical purity of polymer materials, changes in luminous intensity and color purity, and constraints on high-performance blue light polymerization In order to achieve the effect of enhancing the solid-state light emission performance, reducing the quenching effect, and increasing the three-dimensional steric hindrance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
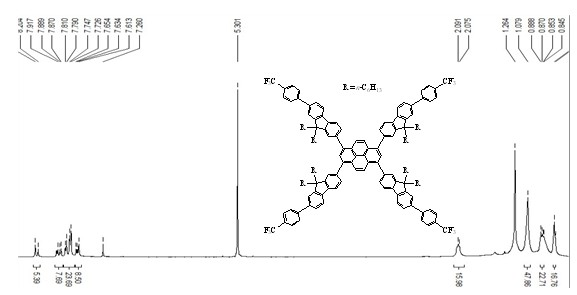
[0039] Target compound H1
[0040] According to the reaction route 1, compound 1 is dissolved in the DMSO solvent, and the compound 2 is obtained after reacting with bromane under room temperature.Compounds 2 in toluene solvents, compound 2 and compound 4- (trifluorophyl) benzene boric acid, and the SUZUKI coupling reaction to the target compound 3 under the condition of catalyst at the condition of catalyst.Finally, compound 3 and compound 4 bromodine 4 bromodramine occurs under the condition that the SUZUKI coupling reaction is reacted to the target compound H1 under the condition of catalyst.
[0041] 【Reaction route 1】
[0042]
[0043]
[0044] (A) bromide, KOH (grinding), Ki, DMSO, room temperature; (B) PD (PPH 3 Cure 4 , 2 m k k 2 CO 3 / H 2 O, toluene;
[0045] (C) n-buli, -78 o C to rt, 12 h; (d) PD (PPH 3 Cure 4 , 2 m k k 2 CO 3 / H 2 O, toluene, microwave.
Embodiment 2
[0047]
[0048] Target compound H3
[0049] According to the reaction route 2, compound 13 is dissolved in the DMSO solvent. After reacting with bromane at room temperature, the compound 14 is obtained.Compounds 14 are dissolved in toluene solvents, and the SUZUKI puppet reaction is obtained under the condition of catalyst with compound 6 under the condition of catalysts in the tetrabenzenyl phosphorus. The compound 15 is obtained.Slowly dripping in a compound 15 add liquid bromine diluted with dichloromethane, which reacts to the compound 16.Compound 10 and compound 16 under the condition that the SUZUKI coupling reaction was obtained by the SUZUKI coupling reaction under the condition of a catalyst.Finally, compound 5 and compound 17 occur in the condition that the SUZUKI couplet reacts occurs under the condition of a catalyst in the catalytic agent of the tetrabenzen phenyl phosphorus.
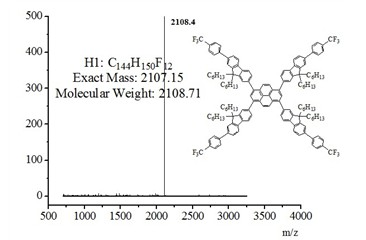
[0023] figure 2 The MALDI-TOF mass spectrometry of the 芘-芴-芴 multi-arm structure materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com