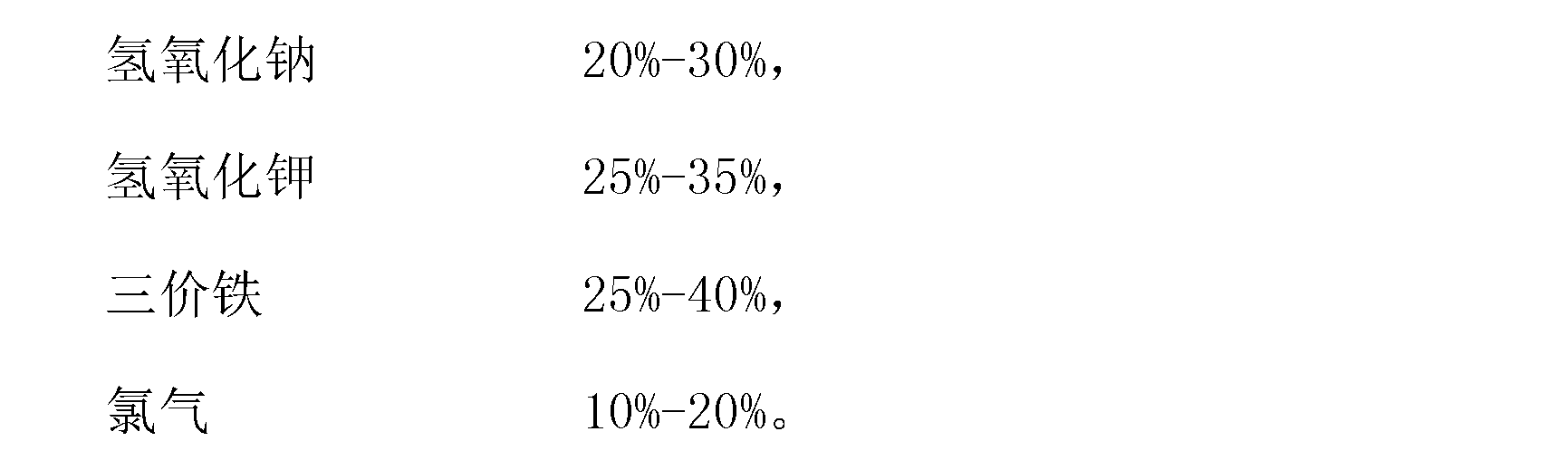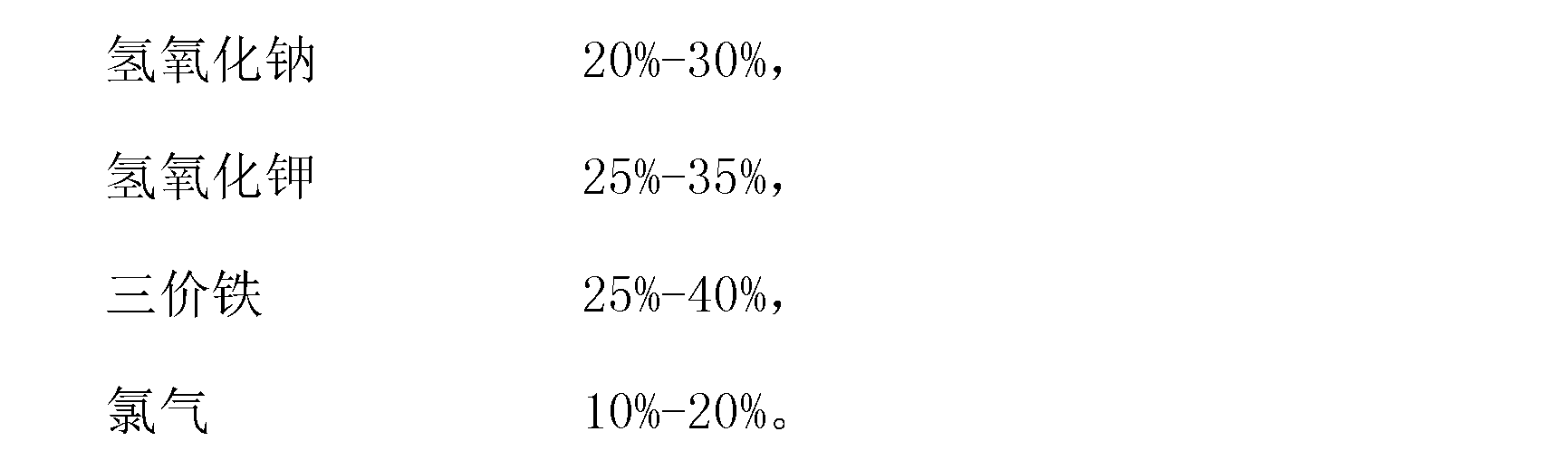Potassium ferrate disinfectant and preparation method thereof
A technology of potassium ferrate and disinfectant, applied in the directions of disinfectants, botanical equipment and methods, chemical instruments and methods, etc., can solve the problems of high cost, large amount, complicated operation and the like, and achieve the effect of reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A potassium ferrate disinfectant is composed of 25 kg of sodium hydroxide, 30 kg of potassium hydroxide, 30 kg of ferric chloride and 15 kg of chlorine gas.
[0020] A preparation method of potassium ferrate disinfectant, comprising the following method steps,
[0021] ①. Sodium production
[0022] React with 40% sodium hydroxide solution and chlorine to generate hyposodium, and then add sodium hydroxide to make saturated sodium hypochlorite, and filter for subsequent use;
[0023] ②. synthesis reaction
[0024] React with saturated hyposodium and ferric iron to generate sodium ferrate;
[0025] ③. Carry out displacement reaction filtration with potassium hydroxide and sodium ferrate to obtain potassium ferrate crude product;
[0026] ④. Washing the crude potassium ferrate with absolute ethanol to obtain the refined potassium ferrate, and then suction filtering and drying at low temperature to obtain the dried refined potassium ferrate.
Embodiment 2
[0028] A potassium ferrate disinfectant is composed of 30 kilograms of sodium hydroxide, 25 kilograms of potassium hydroxide, 35 kilograms of ferric sulfate and 10 kilograms of chlorine gas.
[0029] A preparation method of potassium ferrate disinfectant, comprising the following method steps,
[0030] ①. Sodium production
[0031] React with 40% sodium hydroxide solution and chlorine to generate hyposodium, and then add sodium hydroxide to make saturated sodium hypochlorite, and filter for subsequent use;
[0032] ②. synthesis reaction
[0033] React with saturated hyposodium and ferric iron to generate sodium ferrate;
[0034] ③. Carry out displacement reaction filtration with potassium hydroxide and sodium ferrate to obtain potassium ferrate crude product;
[0035] ④. Washing the crude potassium ferrate with absolute ethanol to obtain the refined potassium ferrate, and then suction filtering and drying at low temperature to obtain the dried refined potassium ferrate....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com