Colloidal gold test paper strip for residue detection of tylosin and tilmicosin, method for using colloidal gold test paper strip and application of colloidal gold test paper strip
A technology of colloidal gold test paper and tylosin, which is applied in the field of veterinary drug residue analysis and immunology, can solve the problems of inconvenient on-site operation and complicated sample processing methods, and achieve small health hazards, fast detection speed, and simple sample processing methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
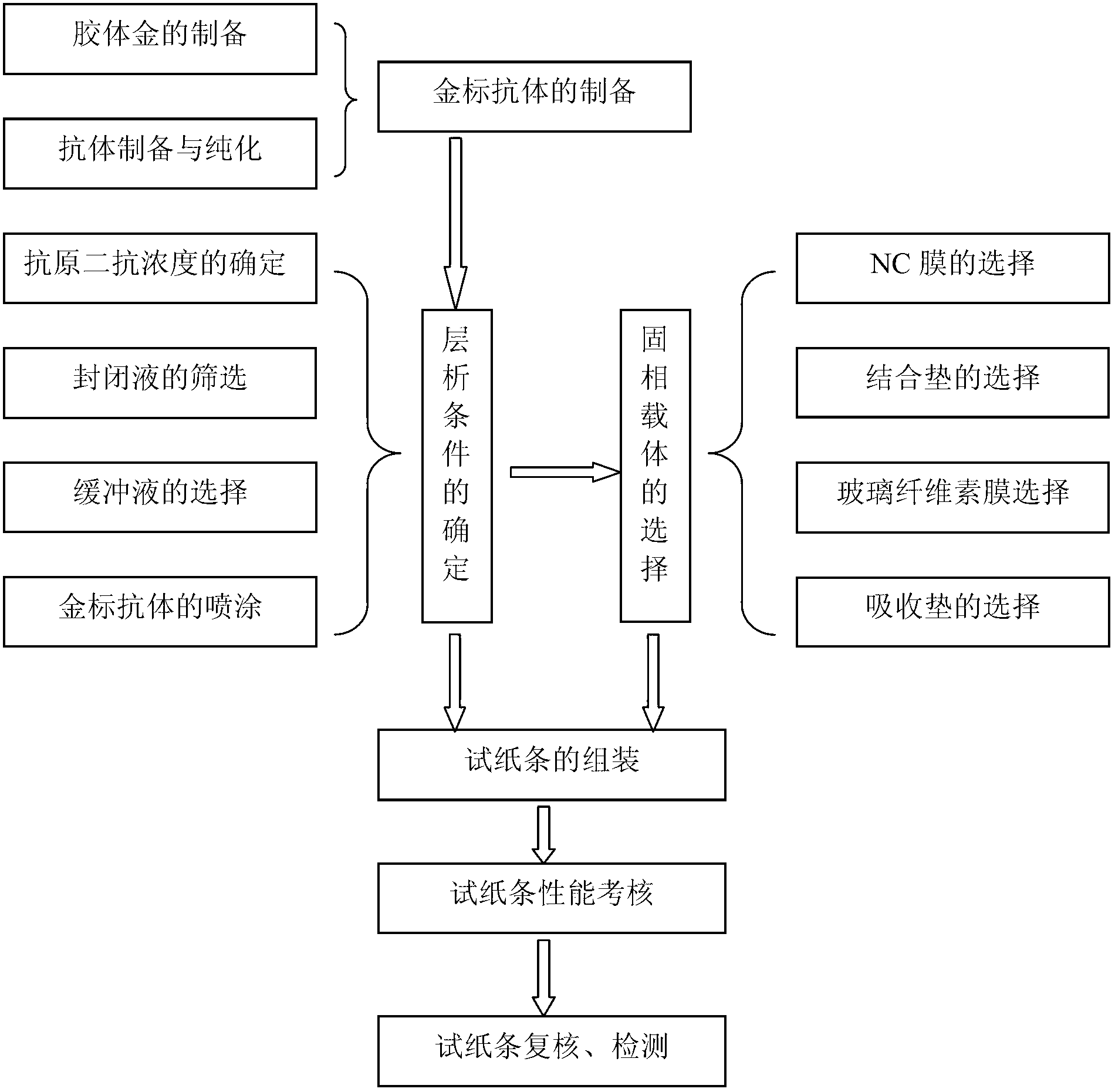
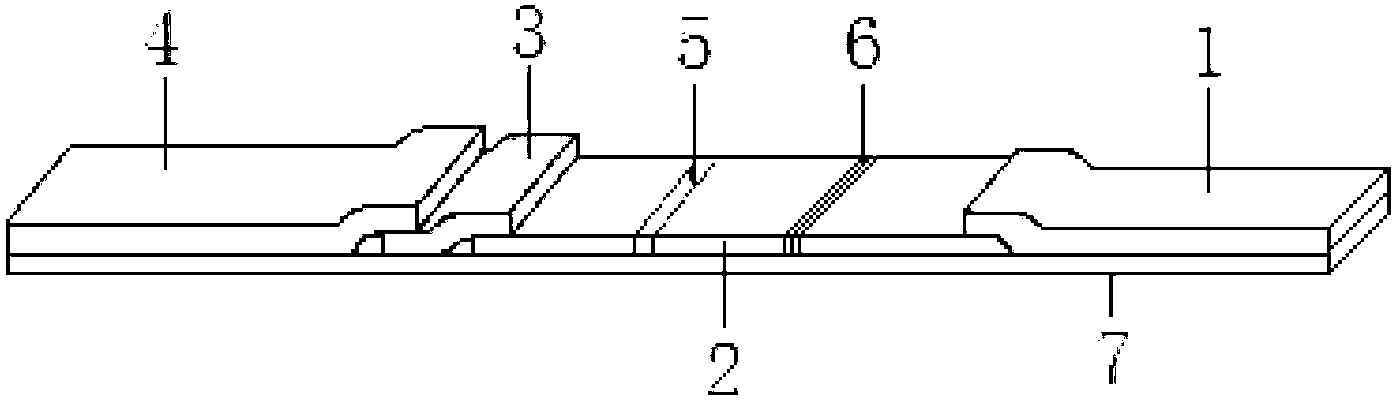
[0024] The preparation of embodiment 1 colloidal gold test strip
[0025] 1.1 Preparation and purification of monoclonal antibodies
[0026] 1.1.1 Preparation of ascites: several Balb / c mice were taken 7 days before inoculation, and each mouse was pretreated by intraperitoneal injection of 0.5ml Freund's incomplete adjuvant. Use RPMI 1640 basal medium to suspend the cells expanded by the hybridoma cell line P3C4 with the preservation number CCTCC NO:C200719, and adjust the number of cells to 1×10 6 cells / mL, each mouse was inoculated with 0.5ml intraperitoneally. Ascites was collected when the abdomen of the mice was obviously enlarged, the spirit became poor, and they were immobile when they were dying.
[0027] 1.1.2 Purification of monoclonal antibody: refer to the method in Zhu Liping's "Common Experimental Methods in Immunology" People's Military Medical Publishing House 2000 Edition: Take 5 ml of the obtained mouse ascites and mix it with an appropriate amount of silic...
Embodiment 2
[0047] The using method and application of embodiment 2 colloidal gold test strip
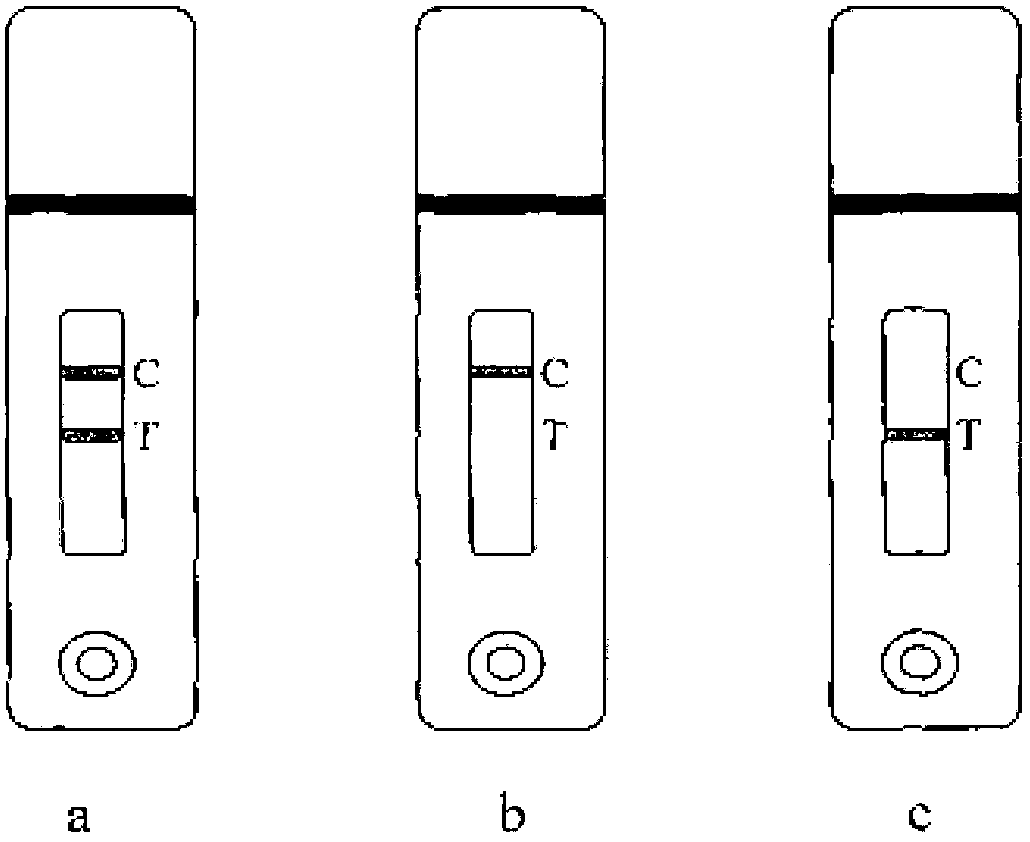
[0048] 2.1 Optimization of colloidal gold test strip detection method
[0049] When the concentration of the coating source is greater than 0.05mg / ml, the test result of tylosin 10μg / L drug is negative; when the coating concentration is less than 0.05mg / ml, the test result of 10μg / L drug is positive, But the detection line of the blank sample is also very light in color. When the concentration of the coating source was 0.05 mg / ml, the result of detecting the concentration of tylosin 10 μg / L was positive, and the negative blank control test line developed a clear color.
[0050] When the quality control line goat or rabbit anti-mouse IgG coating concentration is 0.3mg / ml, the color of the negative blank quality control line is similar to that of the detection line. The final choice of goat or rabbit anti-mouse IgG coating concentration is 0.05mg / ml, and the concentration of the quality control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com