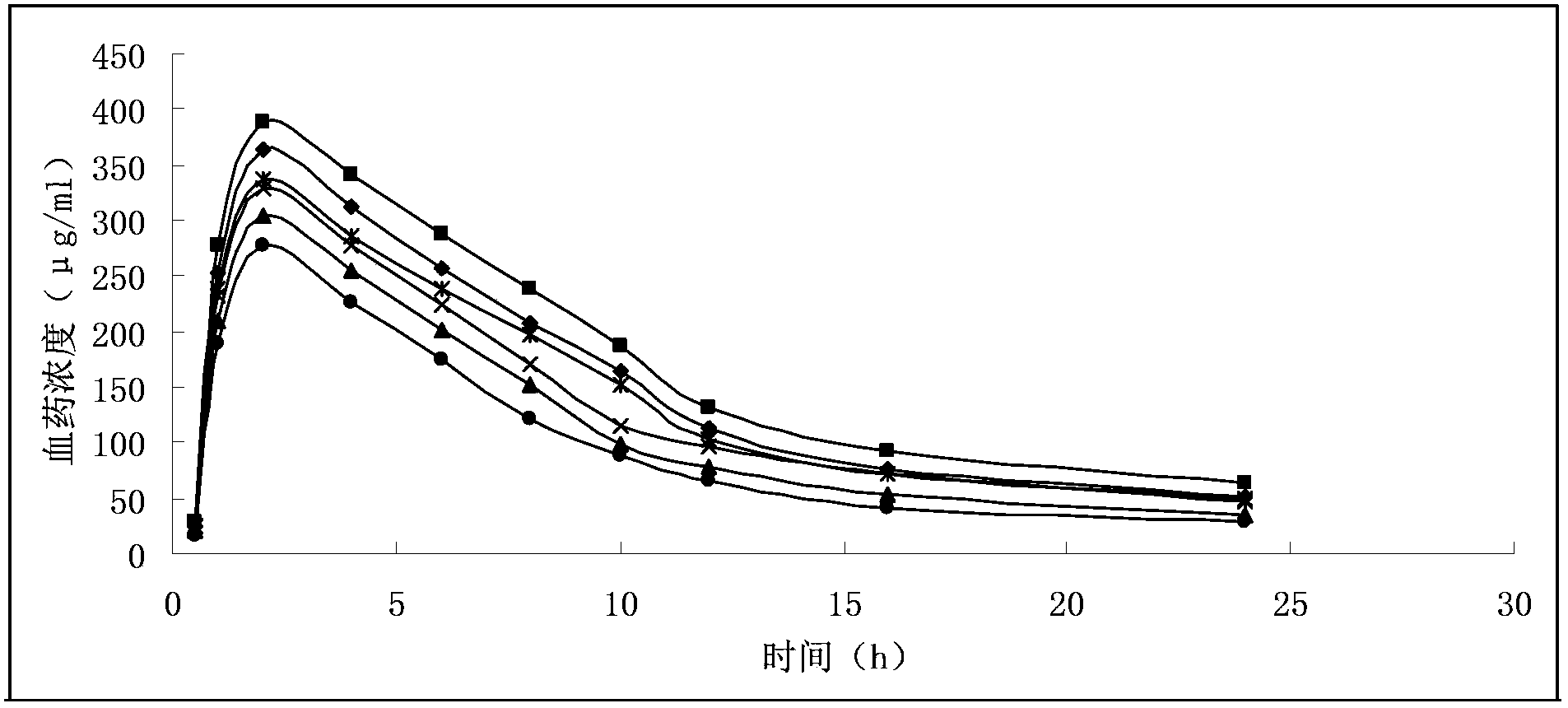
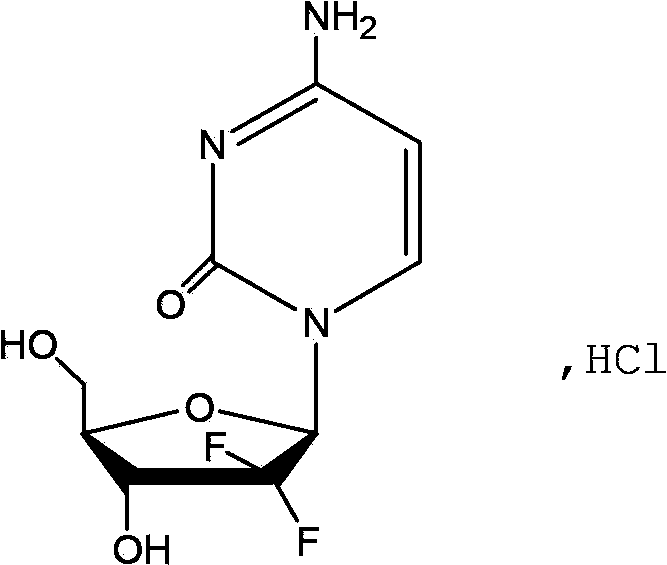
Gemcitabine hydrochloride liposome injection
A technology of gemcitabine hydrochloride lipid and injection, which is applied in the field of medicine, can solve the problems of increased unqualified rate of freeze-dried drugs, negligible stabilizing effect, and low bioavailability, so as to improve bioavailability, good preparation stability, and improve preparation products. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 gemcitabine hydrochloride liposome injection
[0057] The ingredients used and their weights are as follows:
[0058]
[0059] Adopt following process to prepare gemcitabine hydrochloride liposome injection:
[0060] (1) Dissolve 400g of cholesterol, 500g of egg yolk phosphatidylinositol, 100g of lecithin and 100g of Tween 80 in 2000ml of phosphate buffered saline solution with a pH of 7.2 to make blank liposomes;
[0061] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0062] (3) Under sterile conditions, add 200 g of gemcitabine hydrochloride to the liposome in solution state at 60°C, and add 100 g of trehalose, 200 g of mannitol and 40 g of PVP under constant stirring;
[0063] (4) Filtrate with a 0.45 μm microporous membrane, fill, and then freeze-dry. When freeze-drying, the pre-freezing temperature is -45°C, the pre-freezing time is 22 hours, t...
Embodiment 2
[0064] The preparation of embodiment 2 gemcitabine hydrochloride liposome injection
[0065] The ingredients used and their weights are as follows:
[0066]
[0067] Adopt following process to prepare gemcitabine hydrochloride liposome injection:
[0068] (1) Dissolve 800g of cholesterol, 1000g of egg yolk phosphatidylinositol, 200g of lecithin and 300g of Tween 80 in 3000ml of phosphate buffered saline solution with a pH of 7.2 to make blank liposomes;
[0069] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0070] (3) Under sterile conditions, add 200 g of gemcitabine hydrochloride to the liposome in solution state at 60°C, and add 300 g of trehalose, 600 g of mannitol and 60 g of PVP under constant stirring;
[0071] (4) Filtrate with a 0.45 μm microporous membrane, fill, and then freeze-dry. When freeze-drying, the pre-freezing temperature is -45°C, the pre-freezing time is 22 hours, ...
Embodiment 3
[0072] The preparation of embodiment 3 gemcitabine hydrochloride liposome injection
[0073] The ingredients used and their weights are as follows:
[0074]
[0075] Adopt following process to prepare gemcitabine hydrochloride liposome injection:
[0076] (1) Dissolve 2000g of cholesterol, 2500g of egg yolk phosphatidylinositol, 500g of lecithin and 500g of Tween 80 in 5000ml of phosphate buffered saline solution with a pH of 7.2 to make blank liposomes;
[0077] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0078] (3) Under sterile conditions, add 1000g of gemcitabine hydrochloride to the liposome in a solution state at 60°C, and add 1000g of trehalose, 2500g of mannitol and 50g of PVP under constant stirring;
[0079] (4) Filtrate with a 0.45 μm microporous membrane, fill, and then freeze-dry. When freeze-drying, the pre-freezing temperature is -45°C, the pre-freezing time is 22 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com