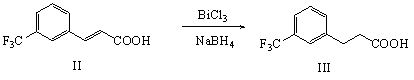Method for preparing cinacalcet hydrochloride
A technology for cinacalcet hydrochloride and hydrochloride, which is applied in the direction of reductive alkylation preparation and organic chemistry, can solve the problems of high price, high production cost, and environmental pollution, and achieve simple operation, low cost, and good purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of embodiment 1 cinacalcet hydrochloride:
[0042] Add 50g (0.231mol) of 3-(trifluoromethyl)cinnamic acid, 250mL of methanol, 30g (0.095mol) of bismuth trichloride, and 29g (0.767mol) of sodium borohydride into the reaction flask, and stir at 20-35°C React for 24 hours, take a sample for thin-layer chromatography analysis, after the reaction is complete, add dichloromethane and wash with a large amount of water, dichloromethane is concentrated and dried to obtain 48g of oily 3-(3-trifluoromethylphenyl)-propionic acid, which is directly used in the following step.
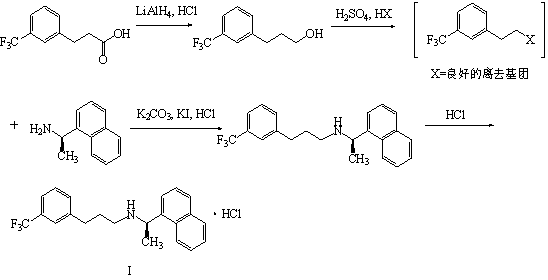
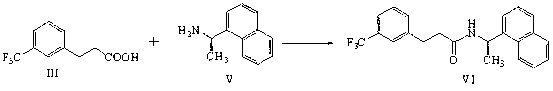
[0043] Add 50g (0.229mol) of 3-(3-trifluoromethylphenyl)-propionic acid, 250mL of dichloromethane and 40g of carbonyldiimidazole into the reaction flask, and add R-1-naphthyl ethyl Amine 29.43g (0.172mol) dichloromethane 250mL solution, after adding 20~35 ℃ stirring reaction for 5 hours, add 250mL water, separate the water layer, wash the organic layer twice with 250mL water, concentrate and dr...
Embodiment 2
[0046] When 3-(trifluoromethyl)cinnamic acid is reduced by sodium borohydride to prepare 3-(3-trifluoromethylphenyl)-propionic acid, 3-(trifluoromethyl)cinnamic acid and bismuth trichloride The molar ratio of sodium borohydride and sodium borohydride is 1.00:0.41~0.50:3.32~4.00 (eg 1.00:0.44:3.40, 1.00:0.45:3.45, 1.00:0.48:3.60), and the temperature is 25~30°C; 3-( When the condensation reaction of 3-(3-trifluoromethylphenyl)-propionic acid and R-1-naphthylethylamine occurs, the moles of 3-(3-trifluoromethylphenyl)-propionic acid and R-1-naphthylethylamine The ratio is 1.00:0.75~1.00 (for example 1.00:0.80, 1.00:0.85, 1.00:0.90, 1.00:1.00), the temperature is 25~30℃; the compound The compound The molar ratio to sodium borohydride is 1.00:4.00~4.92 (for example: 1.00:4.00, 1.00:4.20, 1.00:4.50, 1.00:4.80), and the reaction temperature is 65°C; Sodium oxide adjusts the pH to 6-10. All the other are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com