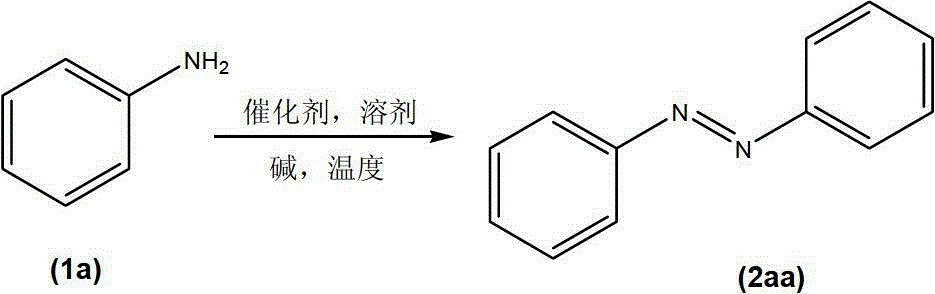
Method for preparing aromatic azoic compound by catalyzing aromatic amine with metal nanocrystals
A technology of metal nanocrystals and azo compounds, applied in chemical recycling, organic chemistry, etc., can solve the problems of non-recycling, poor catalyst universality, and low yield of aromatic azo compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of the Ag nanocrystal that the present invention also provides, it follows the steps:
[0037] 1) In the state of stirring, add silver nitrate salt to octadecylamine at 80°C~100°C to make a solution with a mass ratio of 0.01~0.03g / g;
[0038] 2) In the state of stirring, add the above solution to octadecylamine at 180 ° C ~ 250 ° C for dilution to prepare a solution with a mass ratio of silver nitrate salt to octadecyl amine of 0.003 ~ 0.006 g / g;
[0039] 3) React for 10 to 60 minutes, cool down to 50°C to 80°C, add ethanol and precipitate precipitates, centrifuge and wash with ethanol to obtain Ag nanocrystals, and then disperse the obtained nanocrystals in cyclohexane for later use.
[0040] The present invention also provides the preparation method of described Au nanocrystal, it comprises the following steps:
[0041] 1) Dissolve chloroauric acid tetrahydrate in a mixed solvent of oleic acid and oleylamine at room temperature to make a soluti...
Embodiment 2
[0051] Implementation method: at room temperature, platinum acetylacetonate is added to oleylamine to prepare a solution with a volume concentration of 2 g / L. In the state of stirring, borane-tert-butylamine was added to the above solution to make a solution with a volume concentration of 6 g / L, and then the solution was transferred to a stainless steel autoclave with a polytetrafluoroethylene cup. Then the autoclave was kept at 150° C. for 2 hours and then cooled to room temperature. After adding ethanol, a precipitate was precipitated. After washing with ethanol, Pt nanocrystals were obtained, and then the obtained Pt nanocrystals were dispersed in cyclohexane for subsequent use.
Embodiment 3
[0053] Implementation method: at room temperature, add cobalt acetylacetonate salt into a mixed solvent of toluene and oleylamine to prepare a solution with a volume concentration of 1 g / L. In the state of stirring, borane-tert-butylamine was added to the above solution to make a 3 g / L solution, and then the solution was transferred to a stainless steel autoclave with a polytetrafluoroethylene cup. Then the autoclave was kept at 180 °C for 8 hours and then cooled to room temperature. After adding ethanol, a precipitate was precipitated. After washing with ethanol, Co nanocrystals were obtained, and then the obtained Co nanocrystals were dispersed in cyclohexane for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com