Process for promoting oxidation and crystallization of calcium sulfite produced in carbide slag flue gas desulphurization
A technology of calcium sulfite and process methods, applied in chemical instruments and methods, separation methods, and dispersed particle separation, etc., can solve the problems of no investigation of catalyst reutilization rate, no inspection of oxidation rate, and no attention to the crystal form of desulfurized gypsum. Achieve the effects of shortening crystal growth time, good gypsum quality, and increasing oxidation rate and oxidation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh about 5.0g of calcium carbide slag to fully absorb SO 2 After about 12 hours (hours), prepare a calcium sulfite slurry with a concentration of 0.02mol / L. Use acetic acid to adjust the pH value of the system at 4.0, 4.5 and 5.0, keep the temperature at 50 ° C, and feed air, by Figure 4 It can be seen that the oxidation rates of calcium sulfite in 2 hours were 61.0%, 44.8% and 40.9%, respectively, and the oxidation rates were 0.497% / min, 0.388% / min and 0.342% / min. In the following examples, pH=4.0 is taken as an example for discussion.
Embodiment 2
[0032] Weigh about 2.5, 5.0 and 12.5g of calcium carbide slag to fully absorb SO 2 After about 6, 12 and 30 hours, prepare calcium sulfite slurries with concentrations of 0.01, 0.02 and 0.05 mol / L. Use acetic acid to adjust the pH value of the system at 4.0, keep the temperature at 50°C, and feed air, by Figure 5 It can be seen that the oxidation rates of different concentrations of calcium sulfite in 2 hours were 93.1%, 61.0% and 27.6%, and the oxidation rates were 0.705% / min, 0.497% / min and 0.206% / min, respectively. SEM was used to observe the crystal form of desulfurized gypsum when the concentration of calcium sulfite was 0.02mol / L (no catalyst was added). image 3 a It can be seen that the gypsum is clustered without crystallization, and its quality is poor. In the following examples, the calcium sulfite concentration is 0.02 mol / L as an example.
Embodiment 3
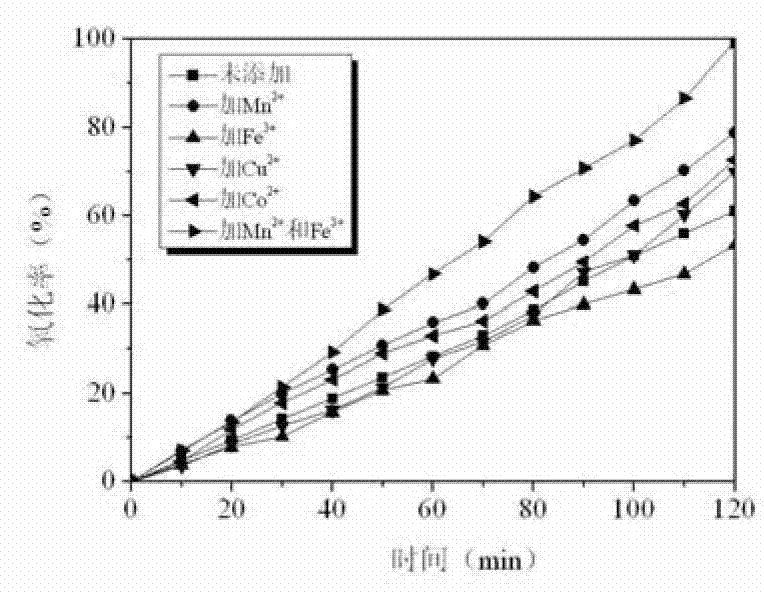
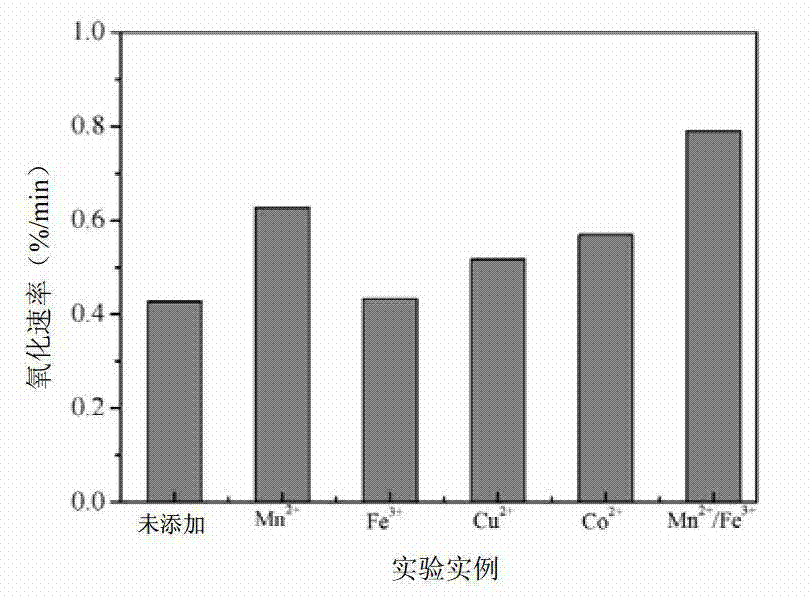
[0034] Weigh about 5.0g of calcium carbide slag to fully absorb SO 2 After about 12 hours, prepare a calcium sulfite slurry with a concentration of 0.02mol / L. Use acetic acid to adjust the pH value of the system at 4.0, keep the temperature at 50°C, blow in air, and add MnSO 4 solution, so that the Mn in the whole system 2+ Concentration is 0.001mol / L, byfigure 1 and figure 2 It can be seen that the oxidation rate of calcium sulfite in 2h is 78.8%, and the oxidation rate is 0.627% / min. Using ICP to test Mn in the filtrate after slurry filtration for desulfurization gypsum 2+ Concentration, when the Mn 2+ The concentration is the initial addition of Mn 2+ 78.2% of the concentration, namely Mn 2+ The remaining rate is 78.2%. It shows that after one use, there is still 78.2% of Mn 2+ Can be recycled. Using SEM observation using MnSO 4 Catalyst desulfurization gypsum crystal form, by image 3 It can be seen from b that the gypsum presents acicular crystal form and is o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com