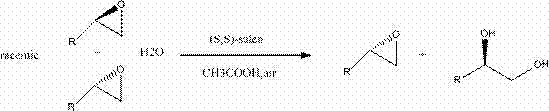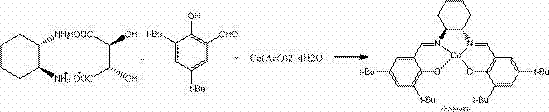Synthetic method of (S,S)-salenCo(II) catalyst and application thereof in split of end epoxide compound
A technology of epoxy compounds and synthesis methods, which is applied in the direction of cobalt organic compounds, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of many process reaction steps, low oxidation yield, environmental pollution, etc., and achieve easy scale The effect of modernized production, wide application prospects and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0027] (1) The filtrate obtained by the resolution of 190.5kg of trans 1,2-cyclohexanediamine to obtain (R,R)-cyclohexanediamine monosalt reaction product filtration, after concentrating, add 400kg ethanol, after being heated to reflux, Add 125kg of D-tartaric acid and 200kg of ethanol solution dropwise, a large amount of solids precipitate out with the addition of tartaric acid, keep warm for 15 hours, cool to 5°C, centrifuge to obtain crude product, recrystallize, and dry to obtain 120kg (S,S)- Cyclohexanediamine monosalt, melting point 280~283°C, specific rotation -12.3°, molar yield 27.2%;
[0028] (2) Put 25kg of (S, S)-cyclohexanediamine monosalt and 21kg of sodium carbonate into a 1000L reactor, add 200kg of ethanol, stir at room temperature for 10 hours, filter, pump the filtrate into a 2000L reactor, and heat to reflux , then add 44.5kg3,5-di-tert-butyl salicylaldehyde and 24kg cobalt acetate to the reaction kettle in turn, add 600kg ethanol, keep warm for 12 hours, c...
Embodiment approach 2
[0031] (1) The filtrate obtained by the resolution of 190.5kg of trans-1,2-cyclohexanediamine to obtain (R,R)-mono-salt reactant and filtered, after concentration, add 500kg tetrahydrofuran, after heating to reflux, dropwise add 125kgD- Tartaric acid and 250kg tetrahydrofuran solution, with the dropwise addition of D-tartaric acid, a large amount of solids precipitated, kept warm for 15 hours, cooled to 5°C, centrifuged to obtain crude product, recrystallized, and dried to obtain 110kg (S,S)-cyclohexane Diamine monosalt, melting point 279~282°C, specific rotation -12.1°, molar yield 24.9%.
[0032] (2) Put 25kg (S, S)-cyclohexanediamine single salt and 26kg potassium carbonate into a 1000L reactor, add 250kg tetrahydrofuran, stir at room temperature for 12 hours, filter, pump the filtrate into a 2000L reactor, and add the filtrate to Reflux, heat to reflux, then add 44.5kg of 3,5-di-tert-butyl salicylaldehyde and 24kg of cobalt acetate into the reaction kettle in turn, add 750...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com