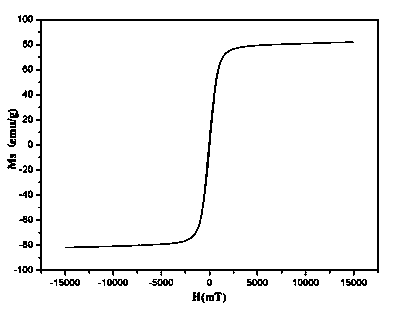
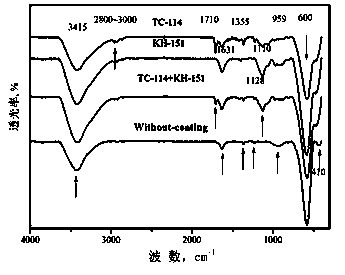
Method for preparing MnZn ferrite
A technology of ferrite and iron salt, which is applied in the field of preparing MnZn ferrite, can solve the problems of uneven microstructure of sintered body, small nanoparticles and easy agglomeration, and low density of green body, so as to improve the density of green body and facilitate dispersion. stability and fluidity, the effect of reducing particle agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the concrete steps of this embodiment are as follows:
[0039] The first step is to prepare MnZn ferrite nanopowder
[0040] Step 1: Weigh 27.802g of ferrous sulfate (0.1mol iron) and dissolve it in 150ml of deionized water, put it in a 500ml beaker for magnetic stirring (stirring speed 300 rpm). Use a graduated cylinder to measure 100ml ammonium bicarbonate (concentration: 0.5mol / L) and 100ml dilute ammonia water (concentration: 5wt%) and pour them into the separating funnel to mix evenly, slowly add dropwise to the ferrous sulfate solution through the separating funnel (dropping The time is 1.5h), and the precipitation of ferrous particles is carried out. At this time, the pH value of the ferrous sulfate solution is adjusted to 7.2, and the ferrous ions are completely precipitated.
[0041] Step 2: Measure 4.2mL H with a graduated cylinder 2 o 2 The solution was added to the suspension obtained in step 1 to transform the ferrous precipitate into the p...
Embodiment 2
[0052] The first step is to prepare MnZn ferrite nanopowder
[0053] Other steps are the same as in Example 1, and the change is that the nominal chemical formula of the MnZn ferrite nanopowder made is Mn 0.7 Zn 0.2 Fe 2.1 o 4 . (0.1mol iron, 0.0095mol zinc, 0.0333mol manganese)
[0054] The second step is to introduce the coupling agent
[0055] Weigh 10 grams of MnZn ferrite nanopowder, then weigh 0.014gTC-114 according to the mass of silicon element as 0.02% of the mass of the nanopowder, and weigh 0.012gKH-151 according to the mass of titanium element as 0.04% of the mass of the powder, Firstly, the two coupling agents were ultrasonically dispersed with 40 mL of absolute ethanol for 20 min, then the weighed nano-powder was added, ultrasonically stirred for 2 h, and dried at 80°C for 2 h to obtain coated modified nano-powder.
[0056] The third step, add binder molding
[0057] Add polyvinyl butyral (PVB) to the powder obtained in the second step, the amount of which...
Embodiment 3
[0061] The first step, preparation of MnZn ferrite nanobody
[0062] Step 1: Weigh 27.083g of ferrous chloride (0.1mol iron) and dissolve it in 150mL of deionized water, place it in a 500mL beaker and perform magnetic stirring (stirring speed 300 rpm). Use a measuring cylinder to measure 100mL ammonium bicarbonate (concentration: 0.5mol / L) and 100mL dilute ammonia water (concentration: 5wt%) into the separating funnel and mix evenly, slowly add dropwise to the ferrous sulfate solution through the separating funnel (drop The time is 1.0h), and the precipitation of ferrous particles is carried out. At this time, the pH value of the ferrous sulfate solution is adjusted to 7.8, and the ferrous ions are completely precipitated.
[0063] Step 2: Measure 5.6mL H with a graduated cylinder 2 o 2The solution was added to the suspension obtained in step 1 to transform the ferrous precipitate into the precursor δ-FeOOH, and the reaction continued for 0.5 h to completely change the color...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com