Technique for synthesizing 2-hydroxy-5-tert octyl acetophenone
A technology of tert-octylacetophenone and process method is applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc. The effect of high yield, high product purity and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
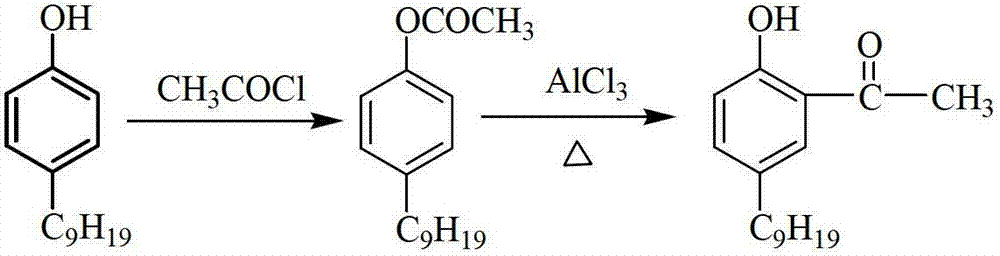
[0029] Add 10.3g of 4-tertoctylphenol (0.05mol) and 30ml of tetrachloroethane into a 250ml four-necked flask equipped with a reflux device (attached with a drying device), a hydrogen chloride absorption device, and a mechanical stirring device, stir, and slowly add 7.9 g (0.1mol) acetyl chloride mixed with 25mL tetrachloroethane solution. After the dropwise addition, the temperature of the system was raised to 80° C., and the stirring was continued, and the reaction was kept for 2 hours, and the color of the solution changed from reddish brown to amber. At this temperature, add anhydrous aluminum chloride (8.1 g in total, 0.06 mol) in batches to the reaction solution, and finish adding in 1 hour. After the aluminum chloride is completely dissolved, add 1 g of acetyl chloride and mix with 3 mL of tetrachloroethane. The temperature of the solution was raised to 110° C., and the reaction was maintained for 10 hours. After the reaction is over, lower the temperature of the reacti...
Embodiment 2
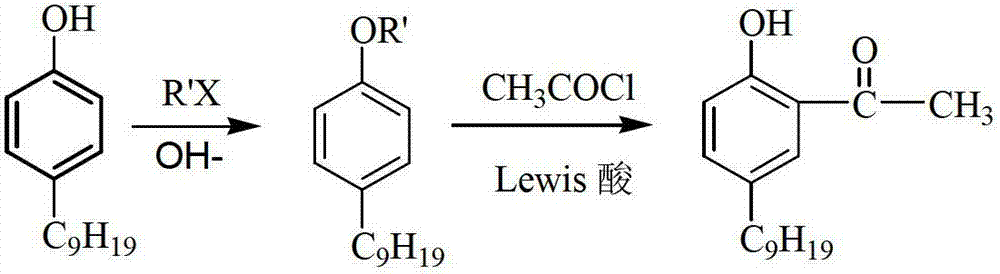
[0031] Add 10.3g of 4-tertoctylphenol (0.05mol) and 30ml of nitrobenzene into a 250ml three-neck flask with a reflux device (attached with a drying device) and a mechanical stirring device, stir, and slowly add 10.2g (0.1mol) of acetic acid Anhydride and 30mL nitrobenzene mixed solution. After the dropwise addition was completed, the temperature of the system was raised to 90° C., the stirring was continued, and the reaction was kept for 3 hours. The color of the solution changed from reddish brown to amber. At this temperature, add anhydrous zinc chloride (total 7.7g, 0.057mol) in batches to the reaction solution, and finish the addition in 1 hour. After the zinc chloride is completely dissolved, add a mixed solution of 2 g of acetic anhydride and 4 mL of nitrobenzene , the temperature was raised to 105° C., and the reaction was maintained for 6 hours. After the reaction is over, lower the temperature of the reaction solution to room temperature, transfer it to a separatory ...
Embodiment 3
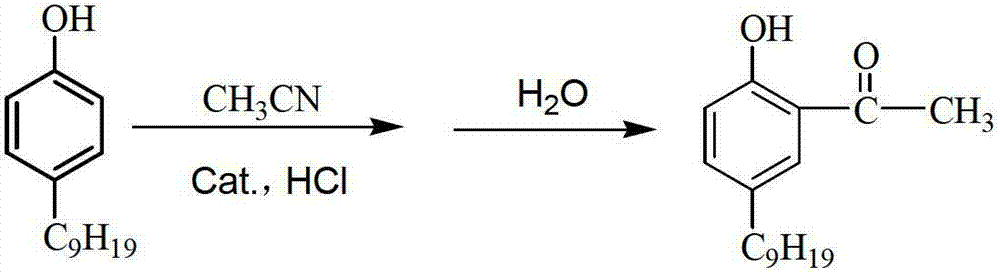
[0033]Add 10.3g of octylphenol (0.05mol) and 30ml of tetrachlorethylene into a 250ml four-necked flask equipped with a reflux device (attached with a drying device), a hydrogen chloride absorption device, and a mechanical stirring device, stir, and slowly add 7.9g (0.1mol ) Mixed solution of acetyl chloride and 25mL tetrachloroethylene. After the dropwise addition, the temperature of the system was raised to 80° C., and the stirring was continued, and the reaction was kept for 2 hours, and the color of the solution changed from reddish brown to amber. At this temperature, titanium tetrachloride liquid (total 11.4g, 0.06mol) was slowly added dropwise to the reaction solution, and after 1 hour, the mixed solution of 1g acetyl chloride and 3mL tetrachloroethylene was added, and the temperature was raised to 110°C, and the temperature was kept React for 10 hours. After the reaction, the temperature of the reaction solution was lowered to room temperature, added 50ml of 0.5mol / L h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com