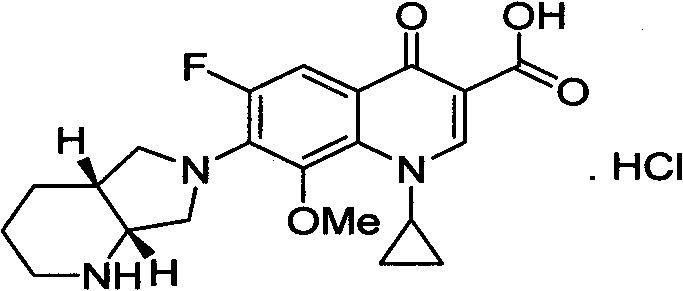
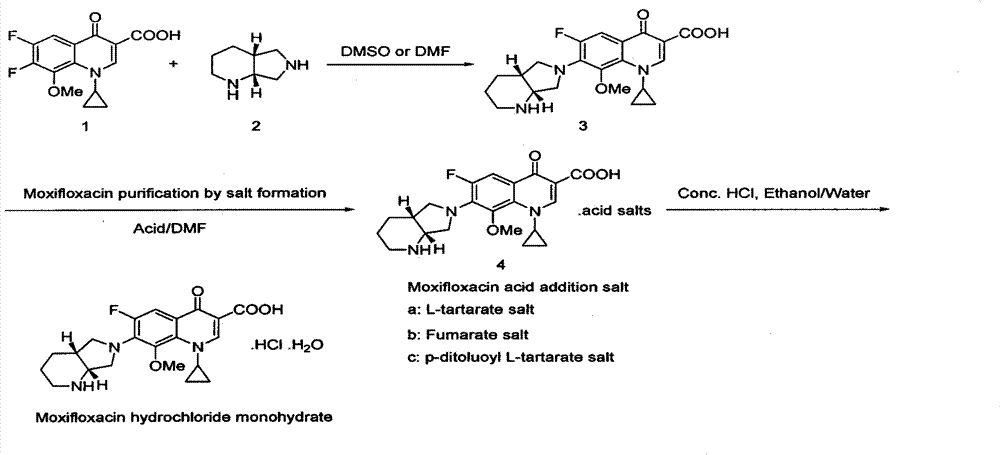
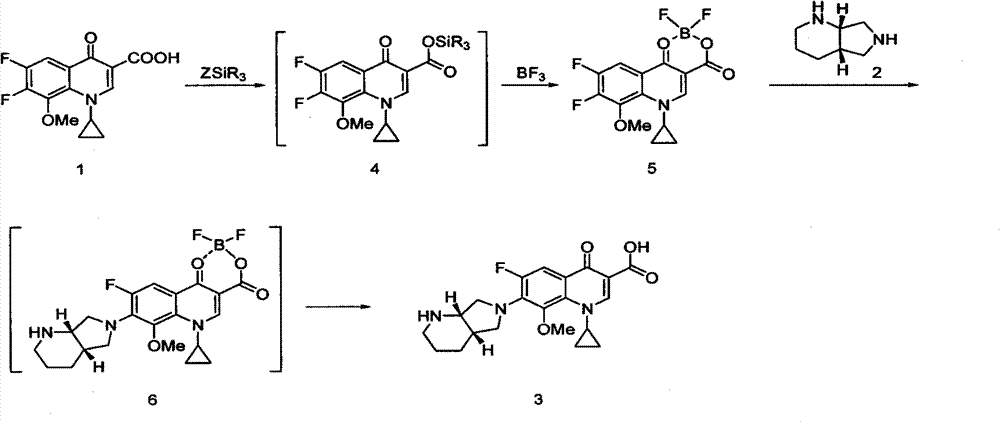
Improvement of preparation method of moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and concentrated hydrochloric acid, applied in the direction of organic chemistry and the like, can solve the problems of restricting the industrial production of moxifloxacin hydrochloride, requiring chiral separation, complicated operation, etc., and achieves reduction of labor intensity, production cost, optical high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of Moxifloxacin Hydrochloride
[0035] In a 2000mL three-neck round bottom flask, add 150.00g of acetic anhydride, stir, heat up to 80°C, slowly add 28.00g of boric acid, stir evenly, slowly heat up to 110°C, and stir for 2 hours. Cool down to 60-70°C, add 100.00 g of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, The temperature was controlled at 80-90° C. to continue the reaction for 2 hours. TLC detects that the reaction is complete, and it is lowered to room temperature. Add acetonitrile 650mL and trimethylamine 367mL to the reaction solution, stir for 30 minutes, add (S, S)-2,8-diazabicyclo[4.3.0]nonane 39.00g ( 1.00eq), heat to reflux for 3 hours, TLC detects that the reaction is complete, cool down to room temperature, add 400mL of methanol, stir for 30 minutes, add 91mL of concentrated hydrochloric acid dropwise under ice bath to control the temperature at 5-10°C, adjust the pH to 1.0, and continue ...
Embodiment 2
[0036] Embodiment 2 Preparation of Moxifloxacin Hydrochloride
[0037]In a 2000mL three-neck round bottom flask, add 150.00g of acetic anhydride, stir, heat up to 80°C, slowly add 28.00g of boric acid, stir evenly, slowly heat up to 110°C, and stir for 2 hours. Cool down to 60-70°C, add 100.00 g of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, Control the temperature at 90-100° C. to continue the reaction for 3 hours. TLC detected that the reaction was complete, cooled to room temperature, added 650 mL of acetonitrile and 592 mL of triethylamine to the reaction solution, stirred for 30 minutes, and added 39.00 g of (S, S)-2,8-diazabicyclo[4.3.0]nonane (1.00eq), heated to reflux for 3 hours, TLC detected that the reaction was complete, lowered to room temperature, added 400mL of methanol, stirred for 30 minutes, added 90mL of concentrated hydrochloric acid dropwise under ice bath to control the temperature at 5-10°C, adjusted the pH to 1....
Embodiment 3
[0038] Example 3 Preparation of Moxifloxacin Hydrochloride
[0039] In a 2000mL three-neck round bottom flask, add 150.00g of acetic anhydride, stir, heat up to 80°C, slowly add 28.00g of boric acid, stir evenly, slowly heat up to 110°C, and stir for 2 hours. Cool down to 60-70°C, add 100.00 g of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, Control the temperature at 90-100° C. to continue the reaction for 3 hours. TLC detected that the reaction was complete, cooled to room temperature, added 650 mL of acetonitrile and 592 mL of triethylamine to the reaction solution, stirred for 30 minutes, and added 39.39 g of (S, S)-2,8-diazabicyclo[4.3.0]nonane (1.01eq), heat to reflux for 3 hours, TLC detects that the reaction is complete, cool down to room temperature, stir for 30 minutes, add 90mL of concentrated hydrochloric acid dropwise under ice bath to control the temperature at 5-10°C, adjust the pH to 1.2, continue to stir and crystallize ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com