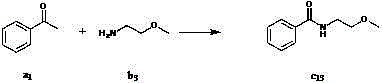
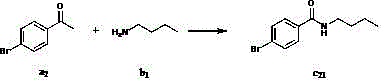Method for preparing amide compound
A technology of amide compounds and substances, which is applied in the field of preparation of amide compounds, can solve the problems of high price, limited large-scale application, and cumbersome catalytic system, and achieves the effects of mild reaction conditions, high compatibility, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
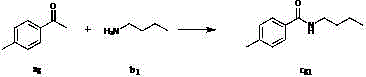
[0027] Add I to the reaction flask sequentially under the reaction condition of zero degrees Celsius 2 (1.5 mmol), compound a 1 (2 mmol), compound b 1 (2 mmol), solvent isopropanol 2mL, after stirring for 5 minutes, the oxidant TBHP (6 mmol) was added. Then the system was stirred for about 12 hours at zero degrees Celsius in the air, then quenched by adding saturated sodium thiosulfate solution, extracted with ethyl acetate (2 mL×3), then adsorbed with 100-200 mesh silica gel, and passed through 300-400 mesh silica gel column eluting to obtain the product c 11 , the yield is 90%. 1 H NMR (CDCl 3 , 400 MHz): δ 7.78 – 7.76 (m, 2H), 7.48 – 7.44 (m, 1H), 7.40 – 7.37 (m, 2H), 6.62 (s, 1H), 3.44 – 3.39 (m, 2H), 1.61 – 1.54 (m, 2H), 1.43 – 1.33 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); 13 C NMR (CDCl 3 , 100 MHz): δ 167.5, 134.7, 131.1, 128.3, 126.8, 39.7, 31.6, 20.1, 13.6; MS (ESI) m / z [M+H] + Calcd for C 11 h 16 NO: 178, found: 178; IR (KBr, cm -1 ): ν 1641. Th...
Embodiment 2
[0029]
[0030] Add (CH 3 ) 4 NI (1.2 mmol), compound a 2 (1 mmol), compound b 1 (2 mmol), solvent dichloromethane 2mL, after stirring for 5 minutes, the oxidant TBHP (6 mmol) was added. After stirring for about 12 hours at zero degrees Celsius in the air, add saturated sodium thiosulfate solution to quench the reaction, extract with ethyl acetate (2 mL×3), then absorb with 100-200 mesh silica gel, and pass through 300-400 The product c was obtained by eluting with a silica gel column 21 , and the yield was 82%. 1 H NMR (CDCl 3 , 300 MHz): δ 7.64 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.2 Hz, 2H), 6.88 (s, 3H), 3.42 – 3.35 (m, 2H), 1.56 – 1.51 (m, 2H), 1.37 – 1.32 (m, 2H), 0.92 (t, J = 7.2 Hz, 2H); 13 C NMR (CDCl 3 , 75 MHz): δ 166.7, 133.5, 131.5, 128.5, 125.7, 39.8, 31.5, 20.0, 13.7; MS (ESI) m / z [M+H] + Calcd for C 11 h 15 79 BrNO: 256, found: 256; C 11 h 15 81 BrNO: 258, found: 258; IR (KBr, cm -1 ): ν 1635. The above detection data confirmed that the...
Embodiment 3
[0032]
[0033] Add (CH 3 ) 3 PhCH 2 N 4 I (1.5 mmol), compound a 3 (3 mmol), compound b 1 (4 mmol), solvent 1,1,1-trichloroethane 2mL, after stirring for 5 minutes, the oxidant TBHP (7 mmol) was added. Then the system was stirred for about 12 hours at zero degrees Celsius in the air, then quenched by adding saturated sodium thiosulfate solution, extracted with ethyl acetate (2 mL×3), then adsorbed with 100-200 mesh silica gel, and passed through 300-400 mesh silica gel column eluting to obtain the product c 31 , and the yield was 83%. 1 H NMR (CDCl 3 , 300 MHz): δ7.71 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 6.89 (s, 1H), 3.42 – 3.36 (m, 2H), 1.57 – 1.52 (m, 2H), 1.37 – 1.32 (m, 2H), 0.92 (t, J = 7.2 Hz, 2H); 13 C NMR (CDCl 3 , 75 MHz): δ166.6, 137.3, 133.1, 128.5, 128.3, 39.8, 31.5, 20.0, 13.6; MS (ESI) m / z [M+H] + Calcd for C 11 h 15 35 ClNO: 212, found: 212; C 11 h 15 37 ClNO: 214, found: 214; IR (KBr, cm -1 ): ν 1630. The above detection d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com