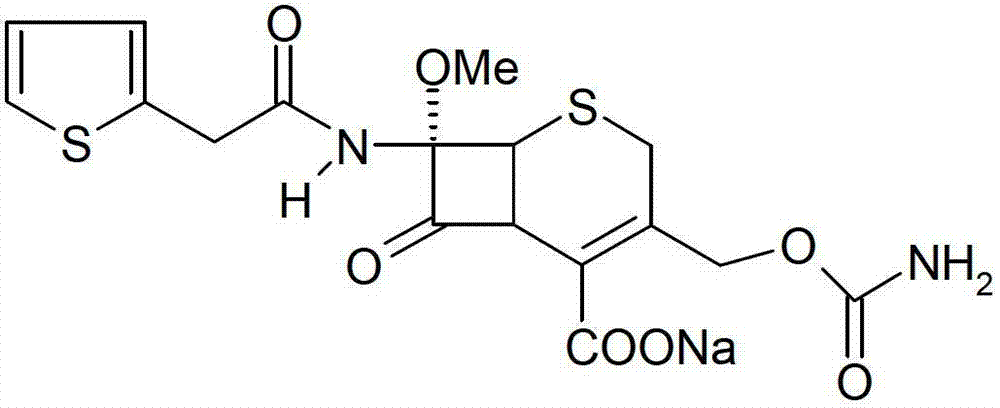
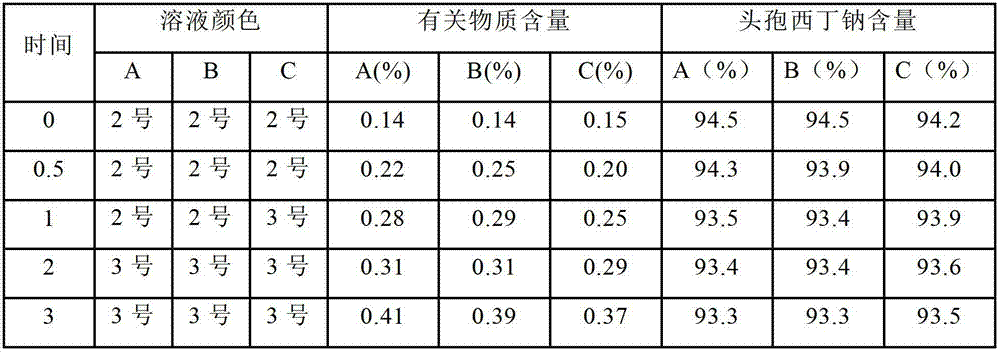
Cefoxitin sodium medicinal composition, powder injection and preparation method thereof
A technology of cefoxitin sodium and a composition, applied in the field of drug invention, can solve the problems of gastrointestinal side reactions, easy increase of impurity content, rapid color change, etc., so as to reduce the incidence of side reactions and improve the stability , the effect of low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The cefoxitin sodium pharmaceutical composition that present embodiment provides, comprises the composition of following weight:
[0031] Cefoxitin Sodium 1000g
[0032] Histidine 30g
[0033] Hydroxypropyl-β-cyclodextrin 80g
[0034] Preparation method: 1) Grind cefoxitin sodium and histidine through a 28-mesh sieve respectively, weigh 1000 g of cefoxitin sodium and 30 g of histidine, mix them evenly, and add them to the coating machine; 2) mix 80 g of hydroxy Propyl-β-cyclodextrin is dissolved in an appropriate amount of ethanol, and the solution is completely and uniformly sprayed onto the surface of the particle layer of cefoxitin sodium and histidine, and vacuum-dried to obtain the cefoxitin sodium pharmaceutical composition.
Embodiment 2
[0036] The cefoxitin sodium pharmaceutical composition that present embodiment provides, comprises the composition of following weight:
[0037] Cefoxitin Sodium 1000g
[0038] Histidine 80g
[0039] Hydroxypropyl-β-cyclodextrin 50g
[0040] Preparation method: 1) Grind cefoxitin sodium and histidine through a 28-mesh sieve, respectively weigh 1000 g of cefoxitin sodium and 80 g of histidine, mix them evenly, and add them to the coating machine; 2) mix 50 g of hydroxy Propyl-β-cyclodextrin is dissolved in an appropriate amount of 70% ethanol aqueous solution (volume percentage concentration), and the above solution is completely and uniformly sprayed on the surface of the particle layer of cefoxitin sodium and histidine, and dried in a fluidized bed. Obtain the cefoxitin sodium pharmaceutical composition.
Embodiment 3
[0042] The cefoxitin sodium pharmaceutical composition that present embodiment provides, comprises the composition of following weight:
[0043] Cefoxitin Sodium 1000g
[0044] Histidine 50g
[0045] Hydroxypropyl-β-cyclodextrin 100g
[0046] Preparation method: 1) Grind cefoxitin sodium and histidine through a 28-mesh sieve respectively, weigh 1000 g of cefoxitin sodium and 50 g of histidine, mix them evenly, and add them to the coating machine; 2) mix 100 g of hydroxy Propyl-β-cyclodextrin is dissolved in an appropriate amount of 50% ethanol aqueous solution (volume percentage concentration), and the above solution is completely and evenly sprayed on the surface of the particle layer of cefoxitin sodium and histidine, and it is obtained by box drying Cefoxitin sodium pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com