Method for preparing 7alpha, 15alpha-dihydroxy androstenone by flowingly addition of hydrogen peroxide
A technology of hydroxyandrostenone and hydrogen peroxide, which is applied in the field of bioengineering and can solve problems such as limited conversion capacity and a large number of toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Place the inoculated slant in a constant temperature incubator at 30°C and culture it for 3 days to obtain mature spores. Inoculate the spores into a 500mL shaker flask containing 100mL seed medium and shake at 28°C and 220rpm. Cultivate on the bed for 4 days to obtain seed culture solution suitable for inoculation.
[0022] (2) Put the seed culture solution into a 250mL shake flask with 30mL fermentation medium at an inoculum amount of 10%, and continue to cultivate under the same conditions for 24 hours to obtain a bacterial fermentation solution;
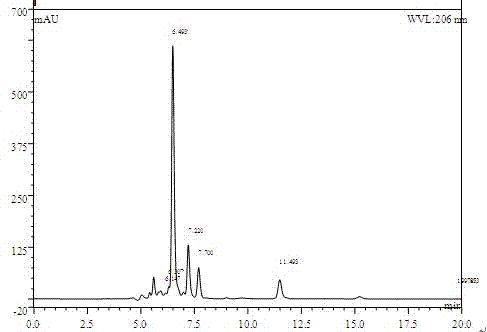
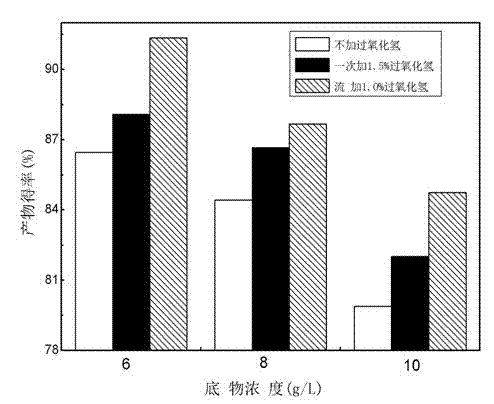
[0023] (3) Put dehydroepiandrosterone into the fermentation broth of the bacteria, the feeding concentration is 8.0g / L, transform under the same conditions for 60 hours and put in the bottle. The extracted product was analyzed by HPLC, and the conversion rate was 84.08%.
Embodiment 2
[0025] (1) Slope culture, seed culture and fermentation culture are the same as in Example 1.
[0026] (2) Put dehydroepiandrosterone into the bacterial fermentation broth, the feeding concentration is 8.0g / L, and at the same time add 1.0% (v / v) hydrogen peroxide with a concentration of 30%. During the transformation process, every 8 Add the same concentration of hydrogen peroxide every hour, stop adding 8-10 hours before putting the bottle, and the conversion time is 52 hours. Take 1mL of fermentation broth to detect, and the conversion rate is 88.21%.
Embodiment 3
[0028] (1) Slope culture, seed culture and fermentation culture are the same as in Example 1.
[0029] (2) Put dehydroepiandrosterone into the bacterial fermentation broth, the feeding concentration is 8.0g / L, and at the same time add 1.5% (v / v) hydrogen peroxide with a concentration of 30%, so that it will no longer be used in the subsequent conversion process Hydrogen peroxide was added, conversion time 52 hours. Take 1mL of fermentation broth to detect, and the conversion rate is 86.39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com