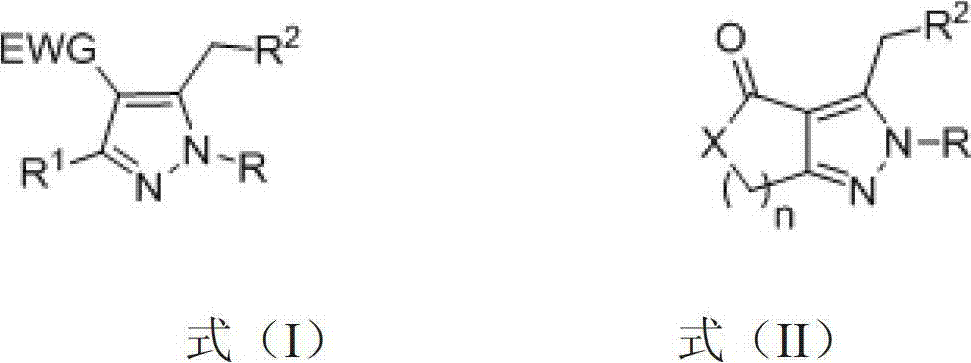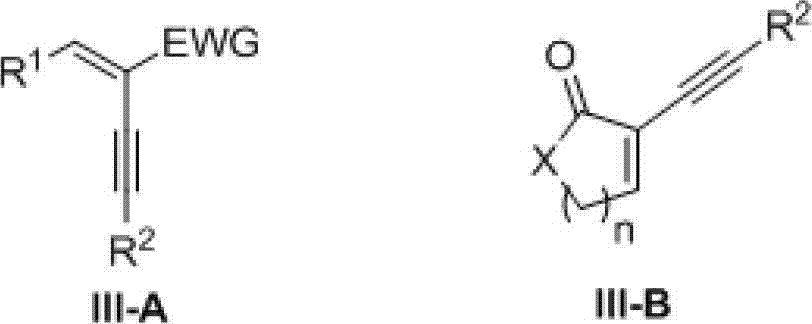Preparation method of electron-deficient group-containing multi-substituted pyrazole derivative
An electron-deficient and multi-substituted technology is applied in the field of preparation of multi-substituted pyrazole derivatives, which can solve problems such as insufficient group compatibility, and achieve the effects of simple operation and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Weigh 3-benzylidene-5-phenyl-4-pentyn-2-one (0.20mmol, 49.2mg), tert-butylnitrile hydrochloride (0.24mmol, 29.9mg), potassium carbonate (0.28mmol , 38.6mg), all of them were added to a small reaction tube, and 2.0ml N,N-dimethylacetamide was added at room temperature. After stirring at room temperature (23°C) for 1.2 hours, the completion of the reaction was monitored by thin-layer chromatography silica gel (TLC), and 4 mL of water and 10 mL of ether were added for extraction, and the aqueous phase was extracted twice with 10 mL of ether. After washing with salt water for 2-3 times, add anhydrous sodium sulfate to dry, filter, the filtrate is rotary evaporated at 20°C to remove the solvent, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:8) to obtain White solid pyrazole derivative pure product IA-1. Yield 97% (64.4 mg). m.p.: 127~128℃.
[0054]
[0055] 1 H NMR (400MHz, CDCl 3 ):δ=7.55(d,J=7.2Hz,2H),7.45~7.37(m,3H),7.26(t...
Embodiment 2
[0057] Weigh 3-(4-methoxybenzylidene)-5-phenyl-4-pentyn-2-one (0.20mmol, 55.2mg), tert-butylnitrile hydrochloride (0.24mmol, 29.9mg ), potassium carbonate (0.28mmol, 38.6mg), all of them were added into a small reaction tube, and 2.0ml of N,N-dimethylacetamide was added at room temperature. After continuing to stir at room temperature for 4 hours, monitor the completion of the reaction by thin-layer chromatography silica gel (TLC), add 4 mL of water and 10 mL of diethyl ether for extraction, and then extract the aqueous phase with 10 mL of diethyl ether twice, combine the organic phases, and wash with saturated saline After 2-3 times, add anhydrous sodium sulfate to dry, filter, the filtrate is rotary evaporated at 20°C to remove the solvent, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:8) to obtain a yellow solid pyridine Azole Derivatives Pure Product IA-2. Yield 85% (62.0 mg). m.p.: 105~106℃.
[0058]
[0059] 1 H NMR (400MH...
Embodiment 3
[0061] Weigh 3-(4-cyanobenzylidene)-5-phenyl-4-pentyn-2-one (0.20mmol, 54.2mg), tert-butylnitrile hydrochloride (0.24mmol, 29.9mg) , potassium carbonate (0.28mmol, 38.6mg), all of them were added into a small reaction tube, and 2.0ml of N,N-dimethylacetamide was added at room temperature. After continuing to stir at room temperature for 1 hour, the completion of the reaction was monitored by thin chromatography silica gel plate (TLC), and 4 mL of water and 10 mL of diethyl ether were added for extraction, and the aqueous phase was extracted twice with 10 mL of diethyl ether. After -3 times, add anhydrous sodium sulfate to dry, filter, and the filtrate is rotary evaporated at 20°C to remove the solvent, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:8) to obtain pyrazole as a white solid Derivatives Pure Product IA-3. Yield 90% (64.3 mg). m.p.: 161~162℃.
[0062]
[0063] 1 H NMR (400MHz, CDCl 3 ):δ=7.73(d,J=8.4Hz,2H),7.69(d,J=8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com