Method for preparing flupirtine maleate capsule
A technology of flupirtine maleate capsules and flupirtine maleate, which is applied in the field of preparation of flupirtine maleate capsules, can solve the problems of gastrointestinal corrosion and not recommend large-scale use, and achieve improved dissolution rate and improved dissolution rate and dissolution rate, the effect is obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of flupirtine maleate capsule, comprises the following steps:
[0032] 1. Weigh and crush 186.2g of calcium hydrogen phosphate and 12.0g of cross-linked polyvinylpyrrolidone through a 100-mesh sieve;
[0033] 2. Weigh 100g (10um) of flupirtine maleate superfine powder after superfine pulverization treatment, and mix it with the above-mentioned auxiliary materials evenly according to the method of equal addition;
[0034] 3. Use an appropriate amount of 50% ethanol solution as a binder to make soft materials, and pass through a 20-mesh sieve to granulate;
[0035] 4. Dry the wet granules at 60°C by blowing air, pass through a 20-mesh sieve, and granulate;
[0036] 5. Add 0.75g of magnesium stearate through a 100-mesh sieve, 0.75g of micropowder silica gel, and mix well;
[0037] 6. Detection of intermediate content;
[0038] 7. Filling, polishing, and blistering to obtain flupirtine maleate capsules.
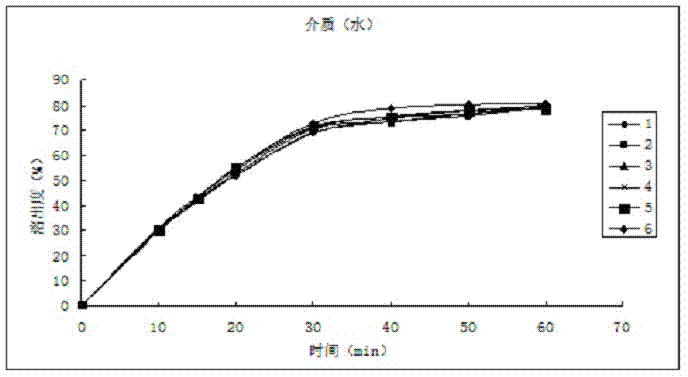
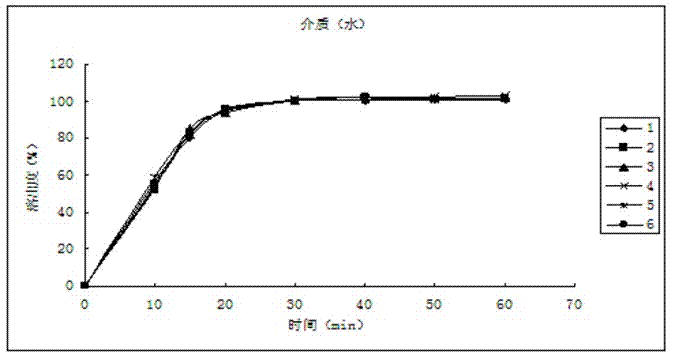
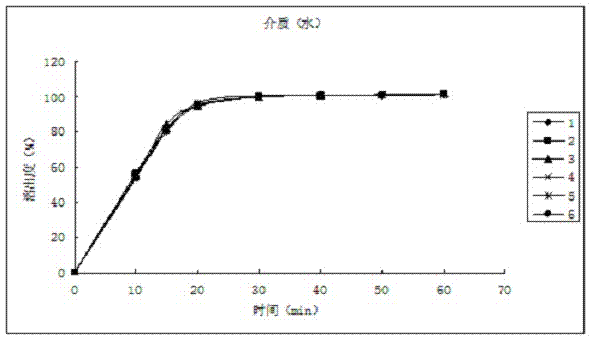
[0039] The dissolution relea...
Embodiment 2
[0042] 1. Weigh and crush 186.2g of calcium hydrogen phosphate and 12.0g of cross-linked polyvinylpyrrolidone through a 100-mesh sieve;
[0043] 2. Weigh 100g (15um) of flupirtine maleate superfine powder after superfine pulverization, and mix it with the above-mentioned excipients evenly according to the method of equal volume addition;
[0044] 3. Use an appropriate amount of 50% ethanol solution as a binder to make soft materials, and pass through a 20-mesh sieve to granulate;
[0045] 4. Dry the wet granules at 60°C by blowing air, pass through a 20-mesh sieve, and granulate;
[0046] 5. Add 0.75g of magnesium stearate through a 100-mesh sieve, 0.75g of micropowder silica gel, and mix well;
[0047] 6. Detection of intermediate content;
[0048] 7. Filling, polishing, and blistering to obtain flupirtine maleate capsules.
[0049] The dissolution release data are as follows:
[0050] time (min) Dissolution rate of No.1 sample (%) Dissolution rate of No. 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com