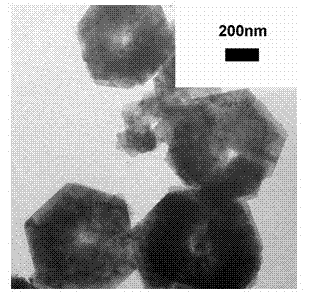
Method for preparing SnS2/SnO2 composite photocatalyst material of numismatics-shaped hollow structure
A composite photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as compound semiconductor materials that have not yet been seen, and achieve excellent heterojunction structure, large specific surface area, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add 5 mmol of SnCl 4 ·5H 2 O was dissolved in 40 mL of acetic acid (10%, volume ratio) aqueous solution; (2) 10 mmol of thiourea powder was added to the SnCl prepared in step (1) 4 ·5H 2 O acetic acid aqueous solution, stirring and dissolving; (3) Put the reaction solution prepared in step (2) into a polytetrafluoroethylene-lined autoclave, seal it and place it in an electric oven, heat at 180°C for 12 hours, stop After heating, cool down to room temperature naturally; (4) Suction filter the precipitate obtained in step (3), wash it with deionized water several times, and then dry it in a vacuum drying oven at 100°C for 3 hours to obtain the coin-shaped hollow structure SnS 2 / SnO 2 Composite photocatalyst materials.
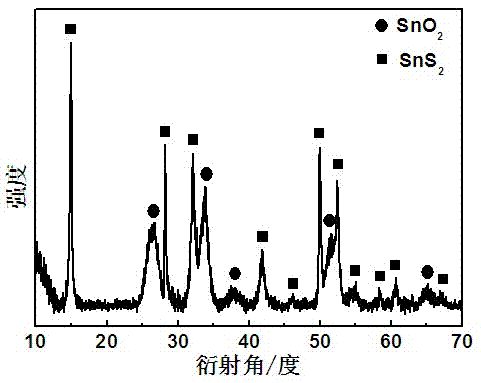
[0024] Such as figure 1 Shown:
[0025] X-ray powder diffractometer (XRD, Cu K α radiation, λ=1.5406?) to determine the crystal phase of the prepared material; the test results show that the hexagonal SnS phase also appears in the XRD pattern ...
Embodiment 2
[0029] (1) Add 5 mmol of SnCl 4 ·5H 2 O was dissolved in 40 mL of acetic acid (10%, volume ratio) aqueous solution; (2) 14 mmol of thiourea powder was added to the SnCl prepared in step (1) 4 ·5H 2 O acetic acid aqueous solution, stirring and dissolving; (3) Put the reaction solution prepared in step (2) into a polytetrafluoroethylene-lined autoclave, seal it and place it in an electric oven, heat at 180°C for 12 hours, stop After heating, cool down to room temperature naturally; (4) Suction filter the precipitate obtained in step (3), wash it with deionized water several times, and then dry it in a vacuum drying oven at 100°C for 3 hours to obtain the coin-shaped hollow structure SnS 2 / SnO 2 Composite photocatalyst materials.
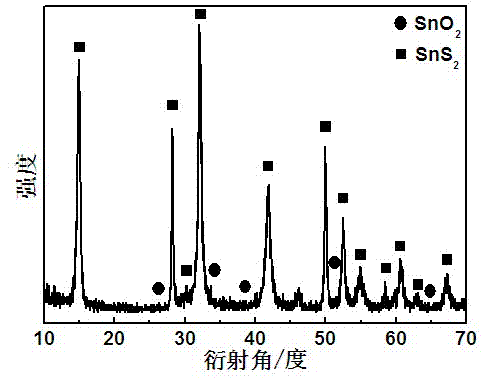
[0030] Such as image 3 Shown:
[0031] X-ray powder diffractometer (XRD, Cu K α radiation, λ=1.5406?) to determine the crystal phase of the prepared material; the test results show that the hexagonal SnS phase also appears in the XRD pattern ...
Embodiment 3
[0035] (1) Add 5 mmol of SnCl 4 ·5H 2 O was dissolved in 40 mL of acetic acid (10%, volume ratio) aqueous solution; (2) 15 mmol of thiourea powder was added to the SnCl prepared in step (1) 4 ·5H 2 O acetic acid aqueous solution, stirring and dissolving; (3) Put the reaction solution prepared in step (2) into a polytetrafluoroethylene-lined autoclave, seal it and place it in an electric oven, heat at 180°C for 12 hours, stop After heating, cool down to room temperature naturally; (4) Suction filter the precipitate obtained in step (3), wash it with deionized water several times, and then dry it in a vacuum drying oven at 100°C for 3 hours to obtain the coin-shaped hollow structure SnS 2 / SnO 2 Composite photocatalyst materials.
[0036] Such as Figure 5 Shown:
[0037] X-ray powder diffractometer (XRD, Cu K α radiation, λ=1.5406?) to determine the crystal phase of the prepared material; the test results show that the hexagonal SnS phase also appears in the XRD pattern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com