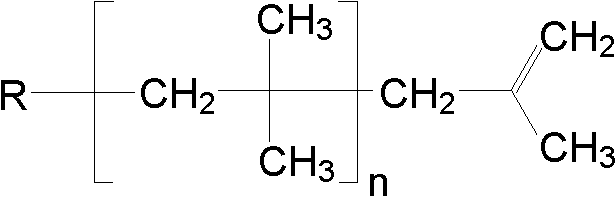
Initiation system for preparation of high reaction activity polyisobutylene and application
A technology of high reactivity and initiation system, which is applied in the field of preparing two series of high reactivity polyisobutenes with low molecular weight or medium molecular weight. Reduce the double bond content and other issues, achieve the effect of stable and controllable cationic polymerization of isobutylene, and increase the molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] At 0°C and under the protection of high-purity nitrogen, add 20 mL of a solution containing isobutene (IB) and dichloromethane (IB=2.0mol L -1 ) in order to add H 2 O, isopropyl ether and SnCl 4 Initiate the polymerization of isobutylene, so that H 2 O / IB=9.5×10 -4 (molar ratio), isopropyl ether / IB=3.3×10 -3 (molar ratio), SnCl 4 / IB=2.6×10 -3 (The molar ratio of). After 30min, add 1mL 0.1g L -1 NaOH / C 2 h 5 OH solution to terminate the reaction. The polymer product was precipitated with ethanol, washed repeatedly with deionized water and ethanol, and finally dried in a vacuum oven at 40°C for 24 hours to remove volatile impurities. The number average molecular weight (M n ) is 1400, the molecular weight distribution index (M w / M n ) was 1.39, and the terminal α-double bond content was 87%.
Embodiment 2
[0039] at 20°C and high-purity N 2 Under protection, dichloromethane, water, isopropyl ether and SnCl 4 Add to the reactor in order to prepare the initiator system solution, in which isopropyl ether / SnCl 4 =0.3,H 2 O / SnCl 4 =0.4, put it for 120h before use.
[0040] The polymerization raw material is the same as in Example 1. Under the protection of high-purity nitrogen at 0°C, the above-mentioned initiator system solution is added to initiate the polymerization of isobutylene, so that isopropyl ether / IB=7.9×10 -4 (molar ratio), H 2 O / IB=9.5×10 -4 (molar ratio), SnCl 4 / IB=2.6×10 -3 (The molar ratio of). After 30min, add 1mL 0.1g L -1 NaOH / C 2 h 5 OH solution to terminate the reaction. The post-treatment method of the polymerized product is the same as in Example 1. Obtained polyisobutene 1.6g, M n for 1900, M w / M n is 2.07, and the terminal α-double bond content is 86%.
Embodiment 3
[0042] The dosage of each component of the initiation system and the preparation steps are the same as in Example 2, except that the preparation temperature is -30°C.
[0043] Initiating system consumption, polymerization raw materials, reaction steps and polymerization product post-treatment method are the same as implementation 2. Obtain polyisobutene 1.5g, M n for 2300, M w / M n is 2.01, and the terminal α-double bond content is 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mn | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com