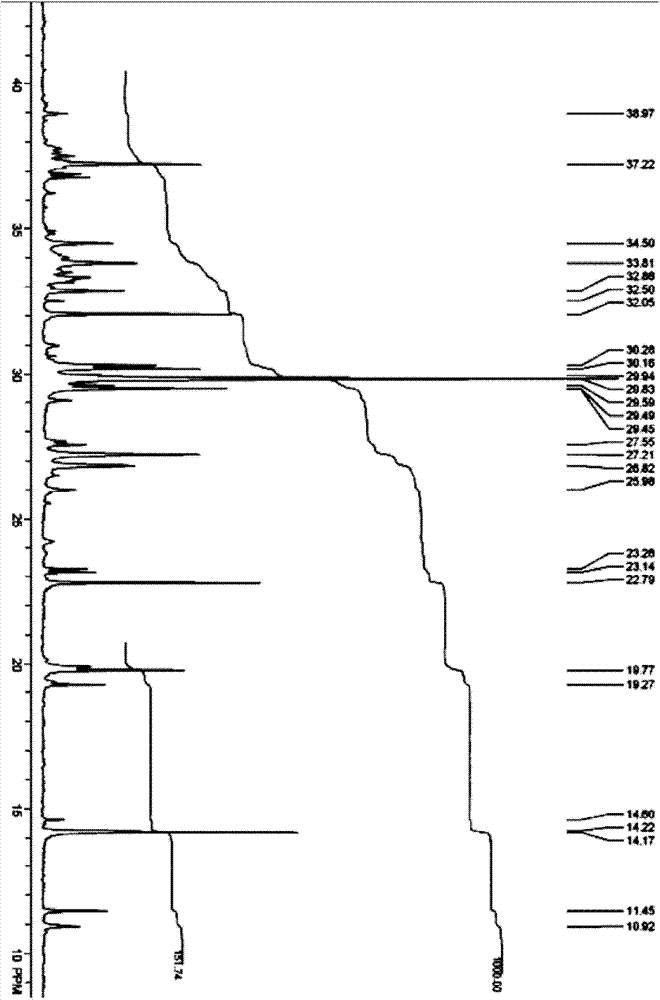
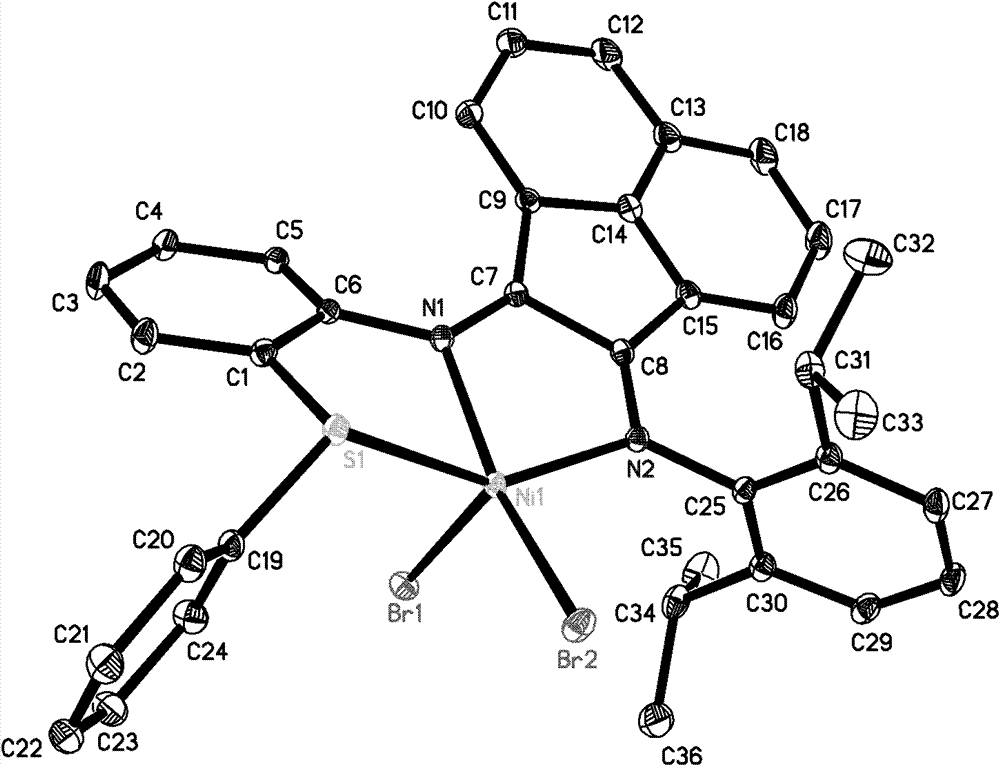
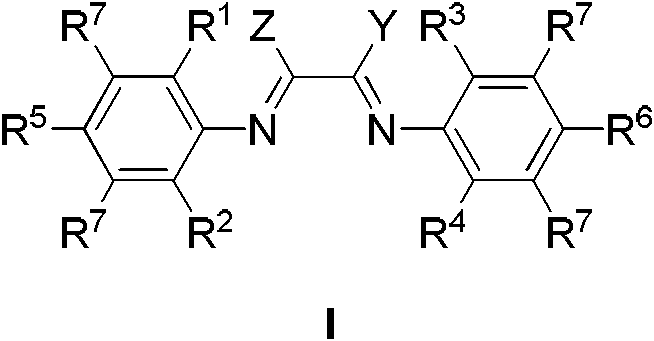
Catalysis systems for preparing highly branched alkane by using olefin
An alkyl and compound technology, applied in the preparation of sulfides, imino compounds, organic chemistry, etc., can solve problems such as inability to meet requirements, lack of methods and catalytic systems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0123] Preparation of Ligand Compounds and Complexes
[0124] The present invention also provides the synthesis of the ligand compound of formula I, comprising steps:
[0125] (a) C is obtained by reacting the corresponding diketone A with an amine compound B.
[0126] (b) Ligand I is obtained by reacting C with amine compound D.
[0127] Described compound A, B, C, D have the following structural formula:
[0128]
[0129] The reaction needs to add 0.001-100% corresponding catalysts to promote the condensation reaction, such as acetic acid, p-toluenesulfonic acid, TiCl 4 , orthosilicate, etc. Specifically, diketone A and amine B are firstly mixed in an inert solvent, and activated by 0.001-100% acetic acid to generate monoimine C, and C continues to react with amine D to obtain the product represented by formula (I). Described inert solvent can be all solvents commonly used in condensation reaction, comprises alcohol, aromatic hydrocarbon, aliphatic hydrocarbon, haloge...
Embodiment 1
[0182] Synthesis of Ligand L1a
[0183]
[0184] In a 100mL egg-shaped flask, add 3.644g (20mmol) of acenaphthoquinone, add 40mL of methanol, add 4.0mL (20mmol) of 2,6-diisopropylaniline, add 2 drops of anhydrous acetic acid, stir at room temperature, and track the reaction by TLC to End, concentrated under reduced pressure, neutral alumina column chromatography, EA: PE = 1: 20-EA: PE = 1: 10 to obtain the orange-yellow product monoimine, the yield is 60%. 1 HNMR (300MHz, CDCl 3 ): δ=8.21(2H, m), 8.01(1H, d), 7.82(1H, t), 7.41(1H, t), 7.27(3H, s), 6.64(1H, d), 2.84(2H, m), 1.18(6H,d), 0.90(6H,d).
[0185] In a 100mL egg-shaped bottle, add 1.708g (5.0mmol) of monoimine, add 40mL of methanol, add 7.5mmol of aniline, add 2 drops of anhydrous acetic acid, stir at room temperature, follow the reaction to the end by TLC, concentrate under reduced pressure, neutral alumina Column chromatography, EA:PE=1:15 gave the orange-yellow product L1a. 1 H NMR (300MHz, CDCl 3 ): δ=7.88-...
Embodiment 2
[0187] Synthesis of Ligand L1b
[0188]
[0189] According to the synthesis method of ligand L1a in Example 1, the second step was to replace aniline with 2,6-dichloroaniline, and the other operating conditions were the same to obtain an orange solid. 1 H NMR (300MHz, CDCl 3 ): δ=8.32(1H, d), 8.10(1H, d), 7.96(1.5H, m), 7.53(2H, d), 7.41(3H, m), 7.38(2H, m), 6.91(0.5 H, m), 6.58 (1H, t), 2.77 (2H, m), 1.29 (2H, d), 0.97 (10H, d), 13 C NMR (75MHz, CDCl3): δ = 162.2, 157.8, 146.1, 133.1, 130.7, 127.5, 124.8, 124.4, 124.3, 123.39, 123.1, 122.7, 120.7, 77.4, 77.6, 28.5, 27.9, 23.5, 22.8. IR(KBr):v(cm -1 )=3052, 2960, 2923, 2865, 1674, 1640, 1602, 1463, 1433, 1242, 1077, 1033, 831, 779, 760, 730; C 30 h 26 Cl 2 N 2 (484.45): Anal. Calc. C 74.22, H 5.40, N 5.77; Found C 73.99, H 5.39, N 5.65.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| bromine number | aaaaa | aaaaa |
| bromine number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com