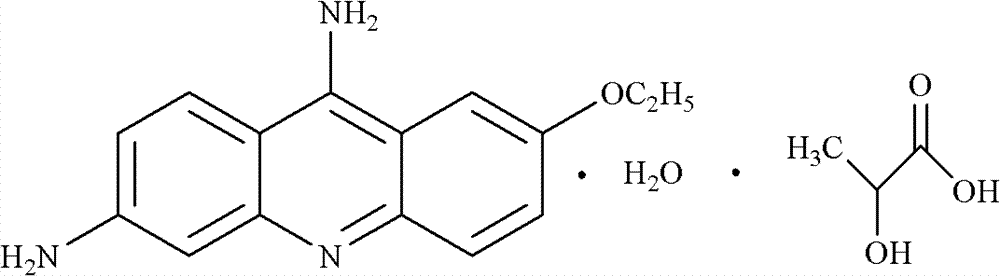
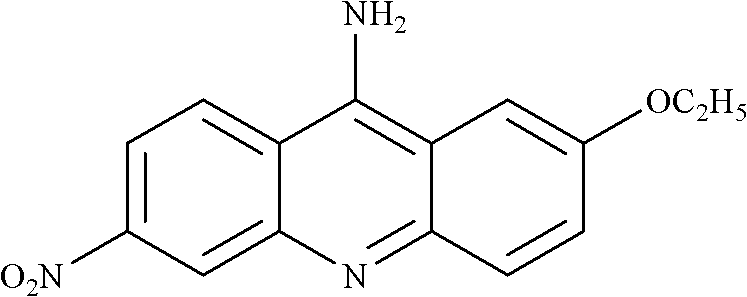
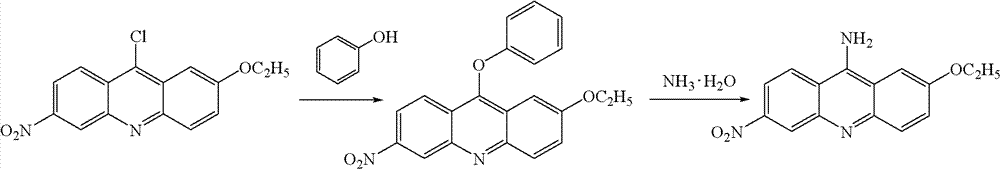
Preparation method of ethacridine lactate intermediate
A technology of ethacridine lactic acid and intermediates, which is applied in the field of preparation of ethacridine lactic acid intermediates, can solve problems such as explosion, and achieve the effects of increasing reaction yield, avoiding environmental pollution, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Add 0.01mol 2-p-ethoxyanilino-4-nitrobenzoic acid and 0.02mol phosphorus oxychloride to a 100ml round bottom flask, reflux at 90°C for 1 hour, add N,N-dimethyl Dimethicone solvent washing, then suction filtration, and then washing with water and suction filtration to obtain 2-ethoxy-6-nitro-9-chloroacridine with a yield of 99%.
[0035] (2) Add 0.01mol 2-ethoxy-6-nitro-9-chloroacridine to a 100ml round bottom flask, add 40ml of N,N-dimethylformamide to dissolve, add 0.01mol of phosphorus oxychloride , 0.02 mol of ethylene glycol, 0.1 mol of ammonia gas was introduced at 0°C, reacted at 100°C for 2 hours, and filtered to obtain 2-ethoxy-6-nitro-9-aminoacridine with a yield of 92%.
Embodiment 2
[0037] In step (1), reflux reaction at 70° C. for 2 h, other steps were the same as in Example 1, and the yield was 95%.
Embodiment 3
[0039] In step (1), reflux reaction at 120° C. for 0.5 h, other steps were the same as in Example 1, and the yield was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com