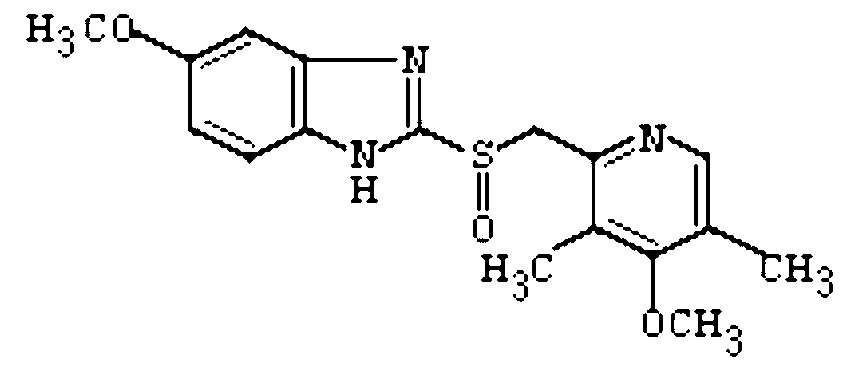
Rapid-release compound omeprazole tablet and preparation method thereof
A technology of omeprazole tablets and omeprazole, which is applied in the direction of pill delivery, digestive system, drug combination, etc., and can solve the problems of highly unstable acid and affecting the rapid treatment of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Tablet core prescription: divided into two specifications of omeprazole 20mg and 40mg, the prescriptions of the two specifications in the tablet core remain unchanged except for omeprazole.
[0031]
[0032] Tablet core preparation method:
[0033] 1. Weigh omeprazole, sodium bicarbonate, magnesium hydroxide, hydroxypropyl cellulose, and croscarmellose sodium according to the prescription amount, add an appropriate amount of water, granulate, and dry at 50°C until the water content is less than 2.0%. , Determination of intermediate content.
[0034] 2. According to the prescription composition and content determination results, convert the dosage of hard sodium fumarate and add it to the dry granules. Mix well and compress into tablets.
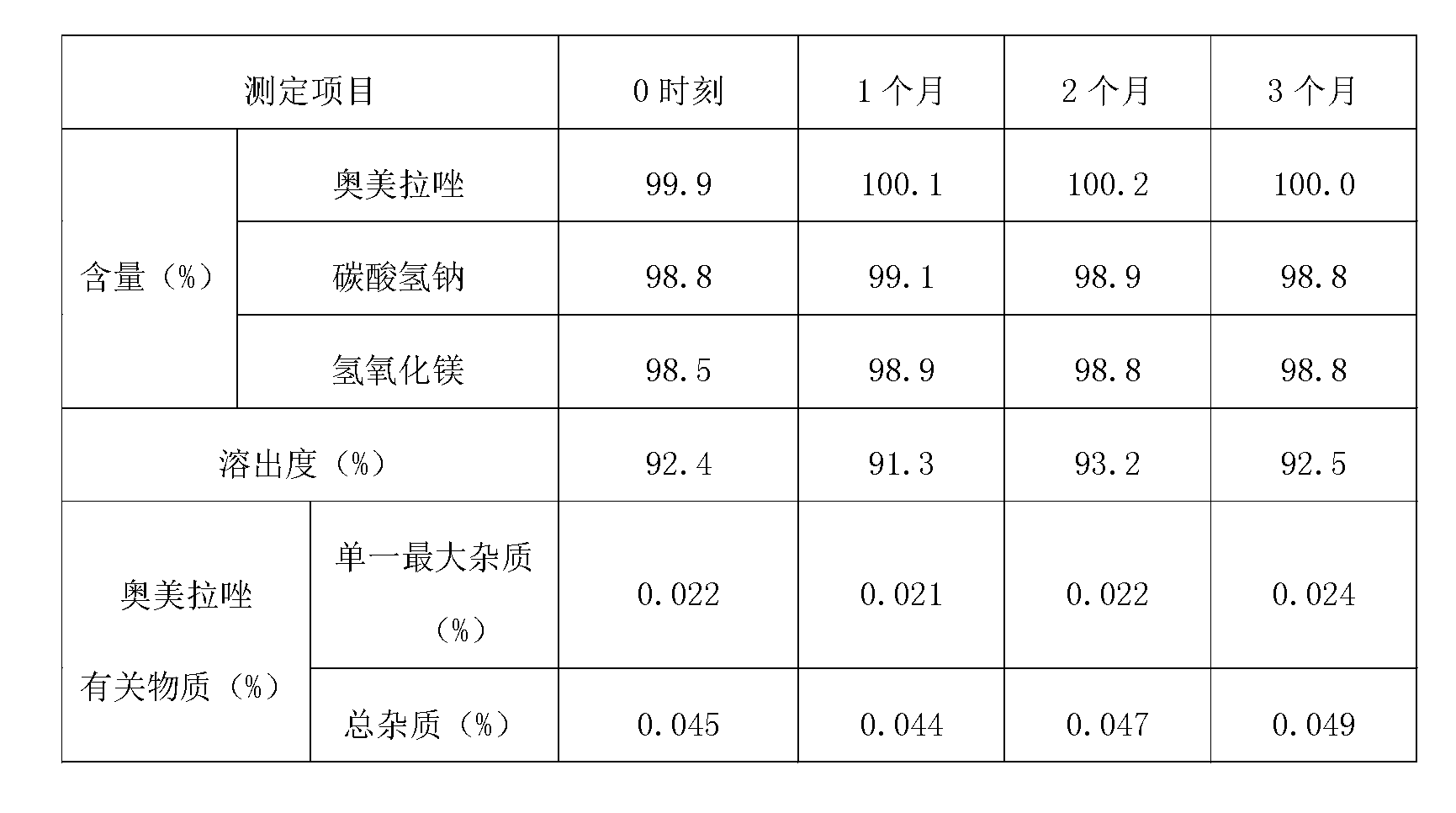
[0035] For the sample prepared in Example 1, measure the content, dissolution rate and related substances of omeprazole, and the content of sodium bicarbonate and magnesium hydroxide. And investigate the increase of omeprazole-re...
Embodiment 2
[0041] Tablet core prescription: same as embodiment 1.
[0042]
[0043] Tablet core preparation method:
[0044] 1. Weigh sodium bicarbonate, magnesium hydroxide and polymer material hydroxypropyl cellulose according to the prescription amount, add appropriate amount of water, granulate, dry at 50°C until the water content is less than 2.0%, crush the granules, and pass through a 60-mesh sieve.
[0045] 2. Take omeprazole and other auxiliary materials according to the prescription amount, mix them with the prepared granules in an equal amount increasing method, and compress into tablets.
[0046] Table 2 Determination results of compound omeprazole tablets (Example 2)
[0047]
[0048] In Example 2, before mixing with omeprazole, first mix sodium bicarbonate and magnesium hydroxide evenly, then mix evenly with hydroxypropyl cellulose, then granulate with water, dry at 50°C, and control the moisture content of the granules to be less than 2.0 %, and ensure that the gra...
Embodiment 3
[0051] Tablet core prescription: omeprazole 20 mg is used as the screening basis for disintegrant dosage.
[0052]
[0053] Tablet core preparation method:
[0054]1. Weigh sodium bicarbonate, magnesium hydroxide and polymer material hydroxypropyl cellulose according to the prescription amount, add appropriate amount of water, granulate, dry at 50°C until the water content is less than 2.0%, crush the granules, and pass through a 60-mesh sieve.
[0055] 2. Take omeprazole and other auxiliary materials according to the prescription amount, mix them with the prepared granules in an equal amount increasing method, and compress into tablets.
[0056] Table 3 Determination results of compound omeprazole tablets (Example 3)
[0057] Measurement items
A
B
C
Dissolution (%)
88.2
92.4
96.6
[0058] In Example 3, further studies were conducted on the amount of disintegrant in the formulation. Croscarmellose sodium is selected as the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com