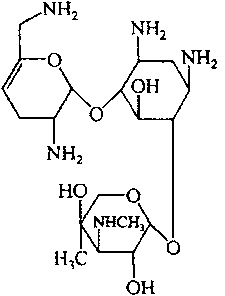
Method for separating and purifying high-purity sisomicin
A sisomycin, separation and purification technology, applied in chemical instruments and methods, chemical recovery, organic chemistry and other directions, can solve the problems of low purity of sisomycin collection solution, low first pass rate, and inability to increase yield, etc. Achieve the effect of saving investment in fixed assets, optimizing purity and yield, and reducing production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
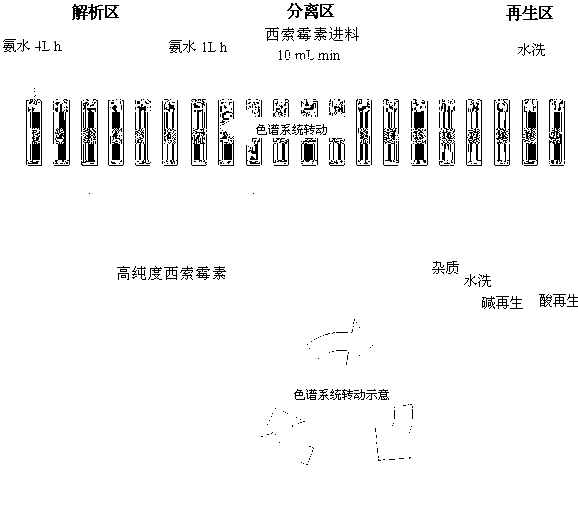
[0055] Combine below figure 1 Embodiment is described in detail:
[0056] The resin selected for the separation chromatography column of the present invention is a strongly basic anion exchange resin, such as 711 resin, the resin aperture is 16-50 mesh, the resin column size is Φ600×800mm, and the actual volume of the resin column is 0.22m 3 , the filling volume of each resin column is 0.18m 3 , the actual filling ratio is 82%. The total size of the system is about 5m×5m×6m (length×width×height), and all separation chromatographic columns are integrated in a continuous chromatographic system that rotates in a forward direction in the form of a carousel.
[0057] The purification process of sisomycin continuous chromatographic separation (20 units) is divided into the following areas:
[0058] (1) Analysis area (1~6 units)
[0059] In the analysis area, the continuous elution method is used, all of which are positively fed, and 2~3N ammonia water is connected in series, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com