Application of bumetanide in inhibition of hepatoma cell transfer
A technology of liver cancer cells and cells, which is applied in the direction of tumor/cancer cells, animal cells, vertebrate cells, etc., can solve the problem of renal tubule ineffectiveness and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
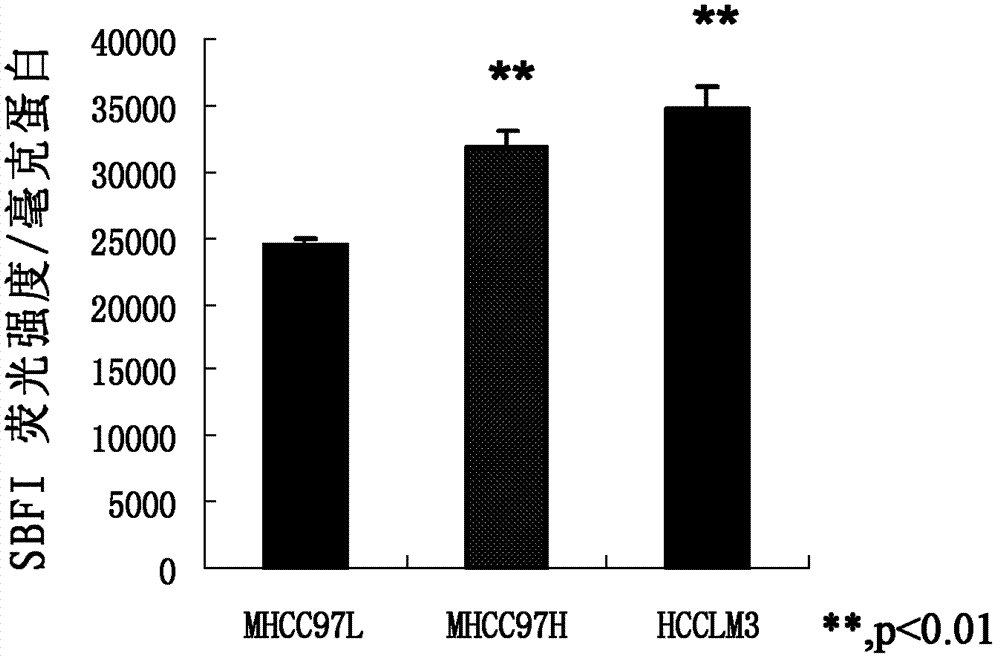
[0031] Example 1. Comparison of the expression of NKCC1 activity in liver cancer cell lines with different metastatic ability
[0032] In this example, the expression of NKCC1 activity in different liver cancer cell lines was compared by detecting the sodium ion content in the protein solution. For the relevant mechanism, see the literature AR. Jayakumar, ML. Liu, M. Moriyama. et al. Na-K-Cl Cotransporter-1 in the Mechani sm of Ammonia-induced Astrocyte Swelling. The Journal of Biological Chemi stry. 2008, 283(49): 33875.
[0033] Perform the following operations on MHCC97L, MHCC97H and HCCLM3 respectively:
[0034] 1. Press the cell 2×10 5 Cells / ml were inoculated on a six-well culture plate, each well was inoculated with 2ml cell suspension, placed at 37℃, 5% CO 2 Culture in an incubator; after the cells have grown to 80-90% confluence, aspirate the liquid and add DMEM high-sugar medium at 37℃, 5% CO 2 Continue to incubate for 30 min in the incubator.
[0035] 2. Aspirate the medium...
Embodiment 2
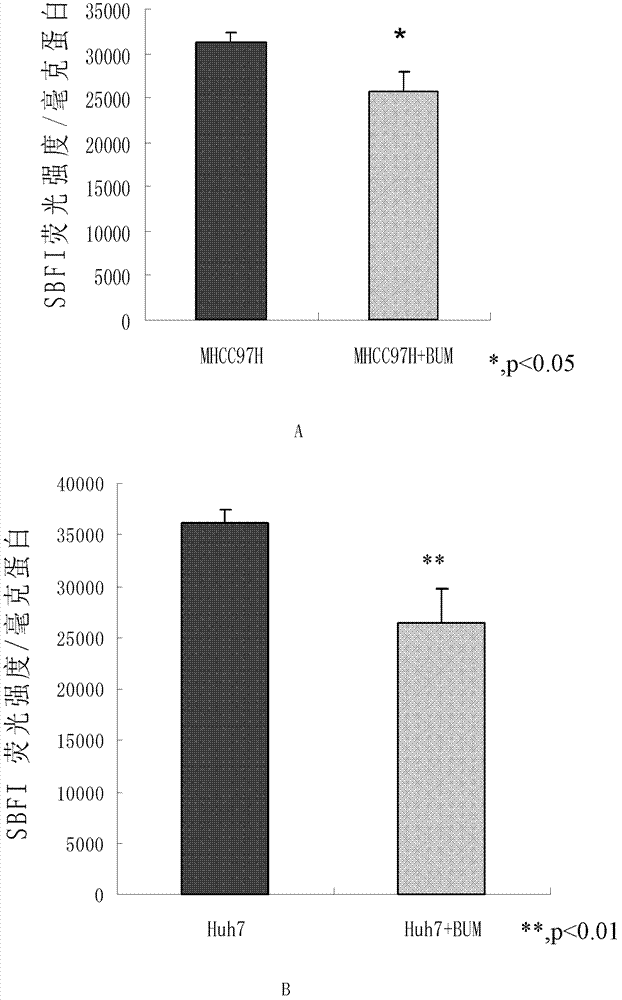
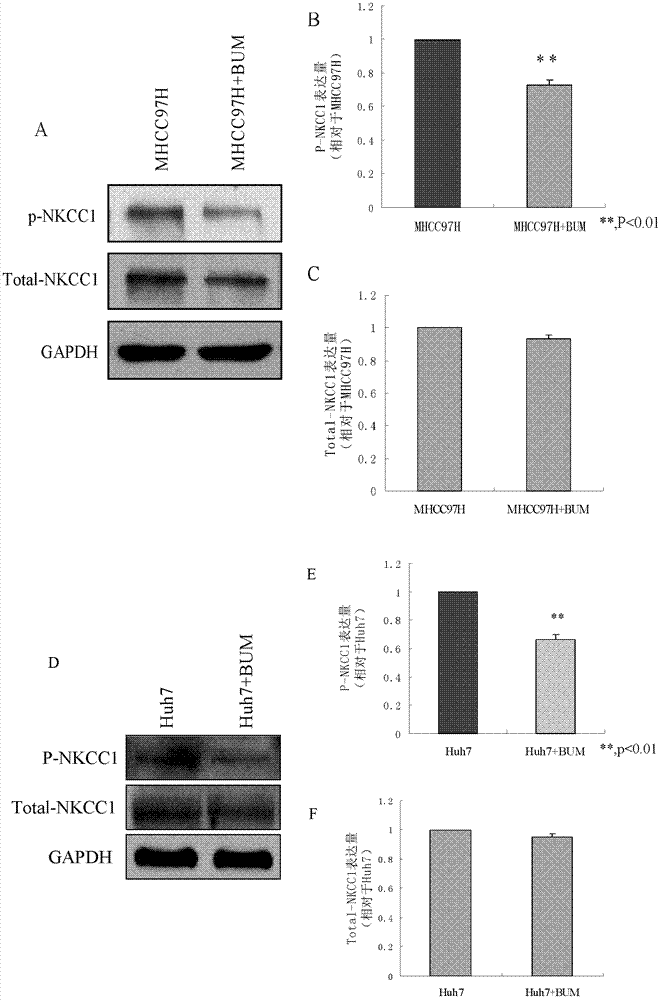
[0039] Example 2. Detection of the effect of bumetanide on the activity of liver cancer cells NKCC1
[0040] 1. Fluorescence spectrophotometer detection
[0041] 1. Liver cancer cell line MHCC97H
[0042] (1) Press 2×10 of the liver cancer cell line MHCC97H 5 Cells / ml were inoculated on a six-well culture plate, each well was inoculated with 2ml cell suspension, placed at 37℃, 5% CO 2 Culture in an incubator.
[0043] (2) After the cells grow to 80-90% confluence, aspirate the liquid and divide into two groups:
[0044] The first group (MHCC97H): add DMEM high sugar medium;
[0045] The second group (MHCC97H+BUM): DMEM high sugar medium containing 50μM bumetanide was added;
[0046] At 37℃, 5% CO 2 Continue to incubate for 30 min in the incubator.
[0047] (3) Aspirate the medium and add DMEM high glucose medium containing 0.05% (volume percentage) pluronic acid and 10μM sodium binding benzofuran isophthalate acetoxy methyl esters-AM (SBFI) at 37℃, 5% CO 2 Incubate for 2h in an incubator p...
Embodiment 3
[0079] Example 3. Effect of bumetanide on the in vitro proliferation ability of liver cancer cell lines MHCC97H and Huh7
[0080] 1. Liver cancer cell line MHCC97H
[0081] 1. Take the hepatocarcinoma cell line MHCC97H in the logarithmic growth phase (70%-80% confluence) and digest the cell suspension with 0.25% pancreatin (Hyclone).
[0082] 2. Divide the wells in the 96-well plate into two groups:
[0083] The first group (MHCC97H): add DMEM high-sugar medium containing 10% (volume percentage) FBS, and then inoculate a cell suspension with a cell concentration of 1000 cells / μl;
[0084] The second group (MHCC97H+BUM): add DMEM high-sugar medium containing 10% (volume percentage) FBS and 50μM bumetanide, and then inoculate cell suspension with a cell concentration of 1000 cells / μl;
[0085] Place at 37℃, 5% CO 2 Incubate in an incubator for 12 hours.
[0086] 3. Use the CCK-8 kit to measure the absorbance of each well at the OD450nm of the microplate reader at 0 days, 1 day, 2 days, 3 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com