Environment-friendly method for preparing adipic acid by catalytic oxidation
A technology of catalytic oxidation and adipic acid, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of difficulty in showing catalytic activity, high reaction temperature, long reaction time, etc., and achieves remarkable results. Catalytic activity, simple preparation method, and the effect of improving the problem of serious deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
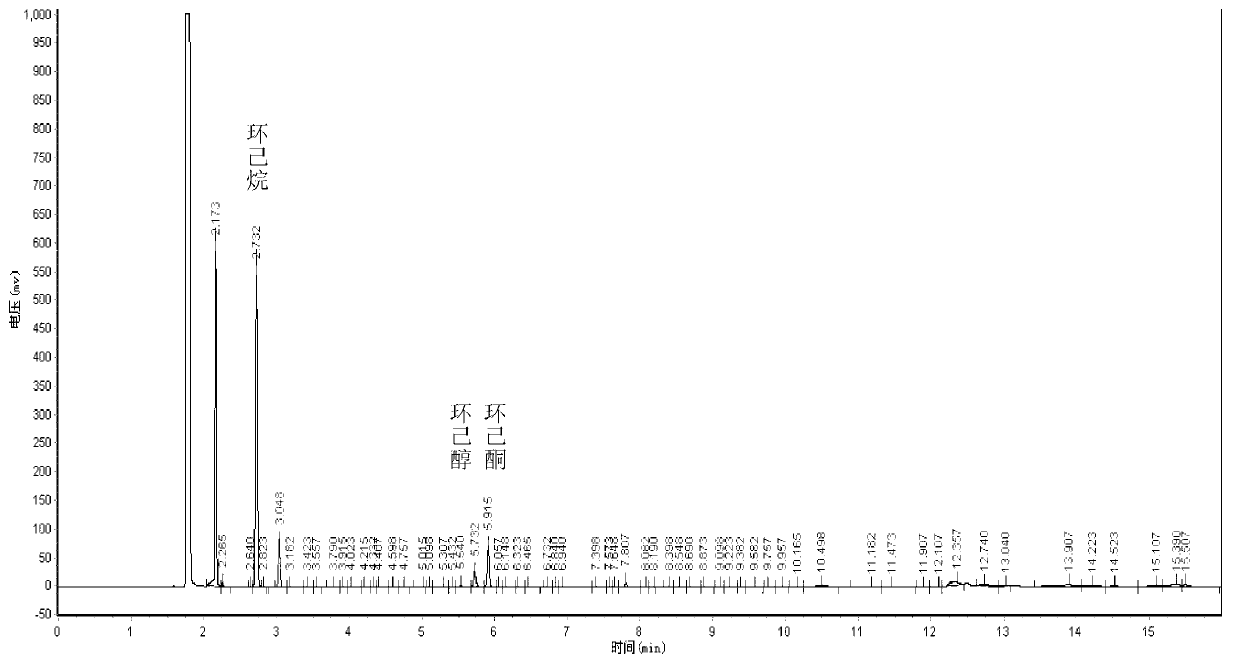
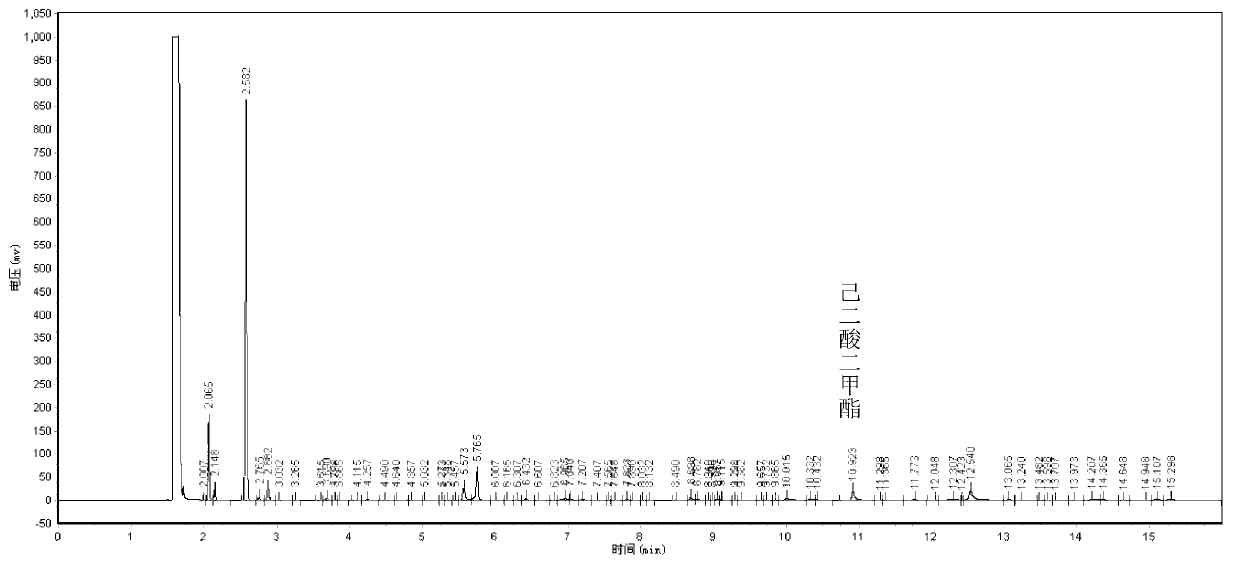
[0024] Add 74mmol cyclohexane, 7ml solvent butanone (the mass ratio of cyclohexane to butanone is 1.1:1), 33mg activated carbon-supported nano-gold catalyst (the mass fraction of Au is 1.25%; the mass fraction of cyclohexane and catalyst Ratio is 188.7:1), 0.7mmol initiator cyclohexanone (mass ratio of cyclohexane to cyclohexanone is 90.6:1), magnetic stirrer, turn on magnetic stirring, and at 130°C, feed oxygen to maintain oxygen pressure At 1.5MPa, react for 8 hours and then cool to room temperature. Activated carbon-supported nano-gold catalyst and reaction solution were obtained by centrifugation, a small part of the reaction solution was used for the result detection, and the remaining part of the reaction solution was extracted three times with deionized water to obtain an oil phase and a water phase, and the water phase was cooled at 0°C for 12 Hours, adipic acid crystalline solids were obtained; the oil phase was vacuum rotary evaporated, the initiator cyclohexanone wa...
Embodiment 2~ Embodiment 8
[0035] Add 74mmol cyclohexane, 7ml methyl ethyl ketone, 33mg catalyst (1.5% gold by weight), 0.7mmol cyclohexanone and a magnetic stirring bar into the reaction kettle, and start the magnetic stirring. At 130° C., feed oxygen to maintain the oxygen pressure at 1.5 MPa, react for 8 hours and then cool to room temperature. Product separation and analysis methods are the same as in Example 1. In Examples 2-8, the mass fractions of gold in the catalysts were 0.5%, 0.75%, 1.0%, 1.25%, 1.5%, 2.0%, and 2.5%, respectively. The cyclohexane conversion and adipic acid selectivity are shown in Table 1.
[0036] Table 1 Quantitative analysis results of Examples 2 to 8 a
[0037]
[0038]a Reaction conditions: 74mmol cyclohexane, 33mg catalyst (1.5% Au), 7ml butanone, 0.7mmol cyclohexanone, 130°C, 1.5MPa, 8h
[0039] b KA oil: Cyclohexanol and cyclohexanone are collectively called KA oil
Embodiment 9~ Embodiment 13
[0041] Add 74mmol cyclohexane, 7ml methyl ethyl ketone, 33mg catalyst (gold mass percentage is 1.5%), 0.7mmol cyclohexanone and a magnetic stirrer to the reaction kettle, turn on the magnetic stirrer, and at a certain temperature, feed oxygen to maintain oxygen The pressure was 1.5MPa, and it was cooled to room temperature after 8 hours of reaction. Product separation and analysis methods are the same as in Example 1. In Examples 9 to 13, the reaction temperatures were 100°C, 120°C, 130°C, 140°C, and 150°C, respectively. The cyclohexane conversion and adipic acid selectivity are shown in Table 2.
[0042] Table 2 embodiment 9 ~ 13 quantitative analysis results a
[0043]
[0044] a Reaction conditions: 74mmol cyclohexane, 33mg catalyst (1.5% Au), 7ml butanone, 0.7mmol cyclohexanone, 1.5MPa, 8h
[0045] b KA oil: Cyclohexanol and cyclohexanone are collectively called KA oil
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com