Heterocyclic group contained amino-methanol derivative, its salt, its synthetic method and its use
A kind of aminomethanol, heterocyclic group technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
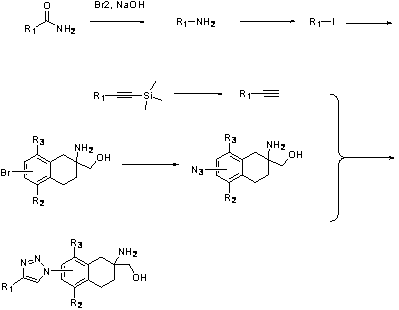
preparation example Construction
[0157] Preparation of Intermediate 1:
[0158]
[0159] The raw material m-bromophenylacetic acid (100 g, 0.47 mol, 1.0 eq) was dissolved in dichloromethane (500 ml), cooled to 0°C in an ice-salt bath, and oxalyl chloride (120 g, 0.95 mol, 2.0 eq ), the dropping temperature was kept at 0°C. After the dropwise addition was completed, the reaction was changed to room temperature for 2 hours. After the reaction was completed, the mixture was concentrated under reduced pressure to obtain m-bromo-phenylacetyl chloride (110 g, 0.47 mol), which was dissolved in dichloromethane (200 ml) for further use.
[0160] In another reaction flask, aluminum trichloride (210 g, 1.9 mol, 4.0 eq ) and dichloromethane (500 mL) were added. Cool the system to -5°C in an ice-salt bath, add the dichloromethane solution of the acid chloride prepared above to the system dropwise, and keep the temperature at -5°C. , continue to pass ethylene gas for 3 hours. After the reaction was completed, it was...
example 1
[0186] Synthesis of Example 1:
[0187]
[0188] Reaction Scheme 6: Synthesis of Example 1
[0189] Preparation of Intermediate 19:
[0190]
[0191] A mixture of methyl 4-methoxybenzoate (33 g, 0.2 mol) and anhydrous hydrazine (7.7 g, 0.24 mmol) was heated to 140 °C under nitrogen, and the reaction was continued at this temperature for 30 min. After cooling to room temperature, the reaction mixture was extracted with ethyl acetate (3 x 100ml). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain the crude product of compound intermediate 19 (30 g), which was directly used in the next reaction.
[0192] Preparation of Intermediate 20:
[0193]
[0194] raw material 18 (0.5 g, 1.0 eq) was dissolved in dry dichloromethane (20 ml), DMF (0.01 g, cat) was added, the temperature was lowered to 0 °C, oxalyl chloride (500 mg, 3.0 eq) was added dropwise, and the React at room temperature for 30 min. After t...
example 2
[0203] Example 2: 6-[5-(4-ethoxyphenyl)-2-[1,3,4]oxadiazolyl]-]-2-amino-1,2,3,4-tetrahydro- 2-Naphthyl-methanol
[0204] NMR (400MHz, CD 3 OD) δ= 8.04 (2H, d), 7.92 (2H, m), 7.41 (1H, m), 7.52 (2H, d), 4.10 (2H, m), 3.60 (2H, d), 3.30 (4H, m), 3.00 (2H, m), 1.40 (3H, t); Rt = 3.34 min, MS (M + H + ): 366, theoretically calculated value: 366
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com