Method for catalyzing synthesizing diisooctyl azelate through load type heteropolyacid
A technology of diisooctyl azelate and heteropolyacid, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve problems such as poor product quality, complicated post-processing, environmental pollution, etc. Achieve the effects of improving esterification efficiency, shortening reaction time, and good demulsification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A method for the catalytic synthesis of diisooctyl azelate by a loaded heteropolyacid, comprising the following steps:
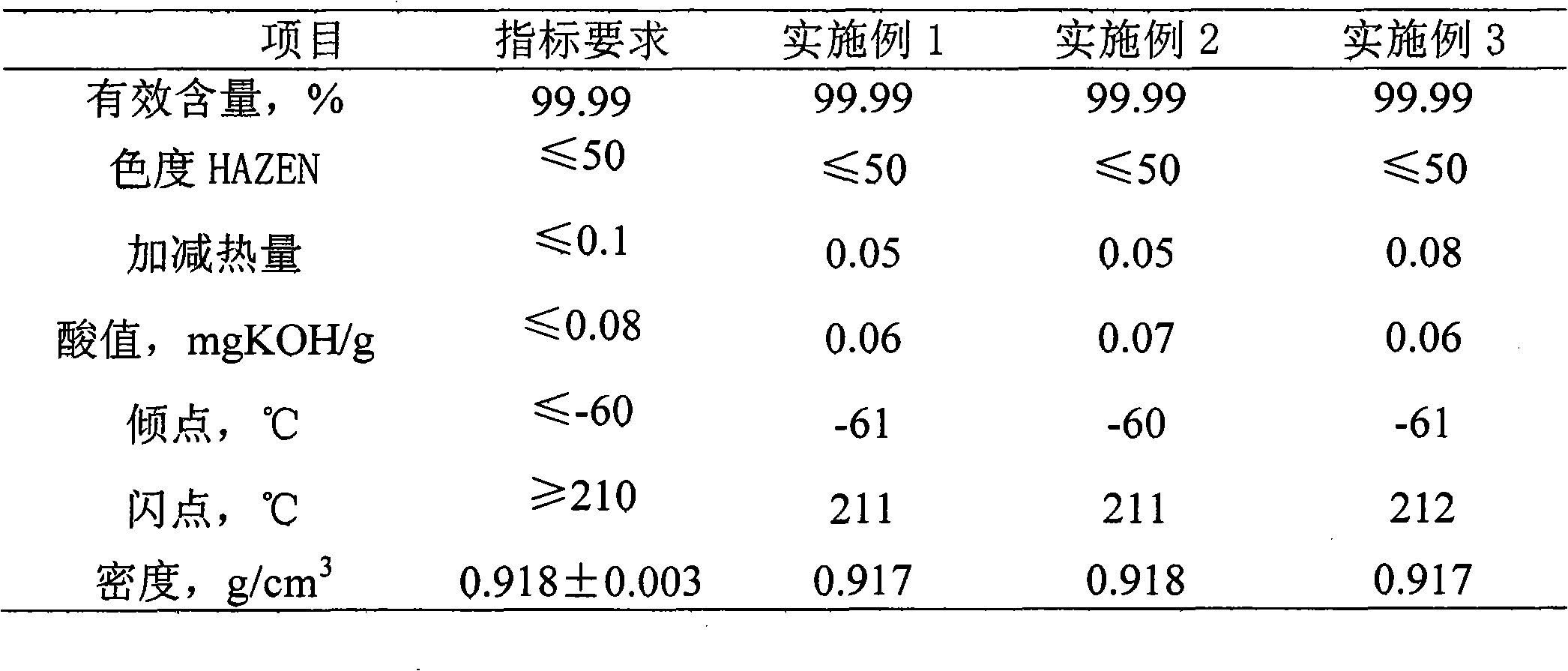
[0019] Under the protection of high-purity nitrogen, mix 100kg of azelaic acid and 260kg of isooctyl alcohol, add 1.0kg of phosphotungstic acid loaded on silica, react at 170°C for 2 hours, then raise the temperature to 200°C for 4 hours to obtain azelaic acid The crude product of diisooctyl ester; the crude product of diisooctyl azelate is passed through a 500-mesh filter plate to remove the catalyst; the filtrate is heated to 170°C, the vacuum degree of the system is kept at 50Pa, and molecular distillation is carried out under reduced pressure for 50 minutes to finally obtain Isooctyl ester finished product. Its performance indicators are shown in Table 1.
Embodiment 2
[0021] Under the protection of high-purity nitrogen, mix 100kg of azelaic acid and 240kg of isooctyl alcohol, add 1.5kg of phosphomolybdic acid loaded on silica, react for 1 hour at 180°C, and react for 3 hours at 210°C to obtain azelaic acid The crude product of diisooctyl ester; the crude product of diisooctyl azelate is passed through a 400-mesh filter plate to remove the catalyst; the filtrate is heated to 185°C, the vacuum degree of the system is kept at 65Pa, and molecular distillation under reduced pressure is carried out for 60 minutes to finally obtain Isooctyl ester finished product. Its performance indicators are shown in Table 1.
Embodiment 3
[0023] Under the protection of high-purity nitrogen, mix 100kg of azelaic acid and 240kg of isooctyl alcohol, add 1.8kg of phosphomolybdic acid loaded on titanium dioxide, react at 160°C for 3 hours, and raise the temperature to 230°C for 1 hour to obtain azelaic acid diiso The crude product of octyl ester; the crude product of diisooctyl azelate is passed through a 600-mesh filter to remove the catalyst; the filtrate is heated to 200°C, the vacuum degree of the system is kept at 65Pa, and molecular distillation is carried out under reduced pressure for 60 minutes to finally obtain diisooctyl azelate Ester products. Its performance indicators are shown in Table 1.
[0024] Table 1 Diisooctyl azelate Performance Index
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com