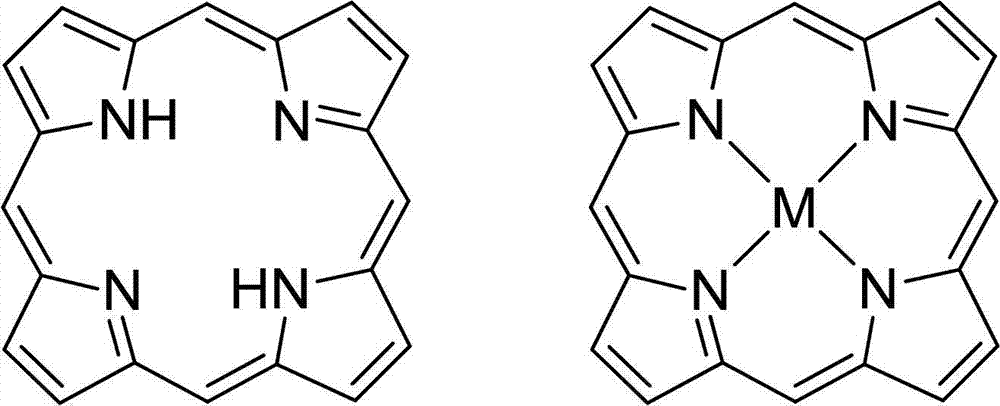
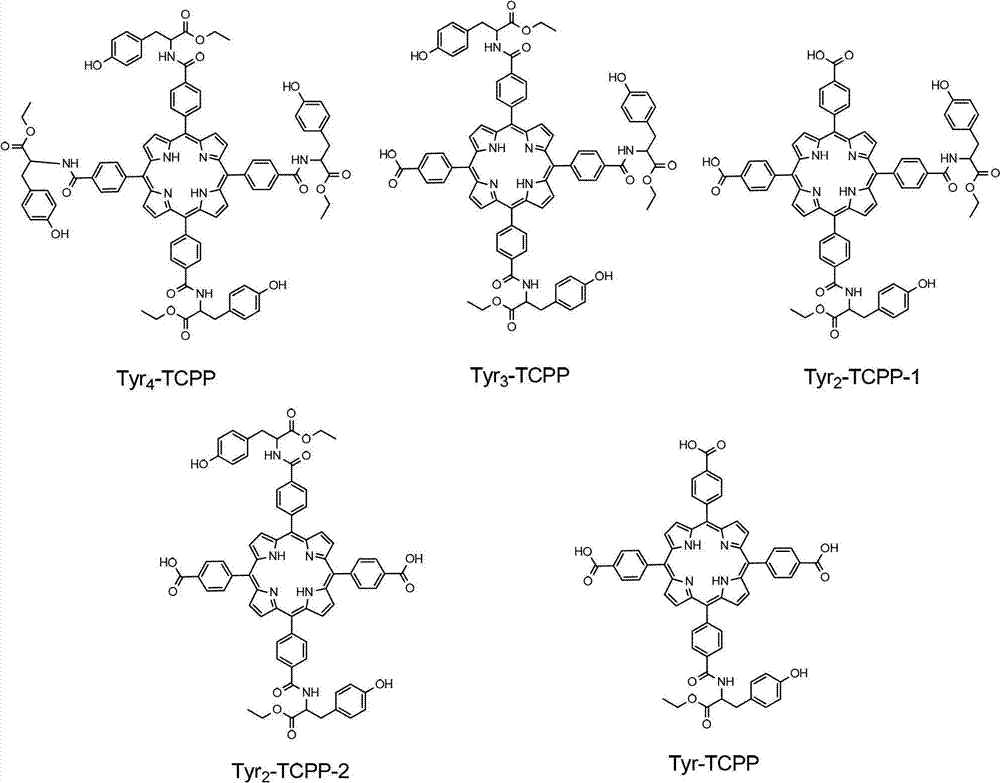
Novel porphyrin ligand and metal complex, preparation method and application for novel porphyrin ligand
A technology of metal complexes and porphyrins, applied in chemical instruments and methods, photosensitive equipment, porphine/acridine porphine, etc., to achieve the effect of cheap and easy-to-obtain raw materials and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
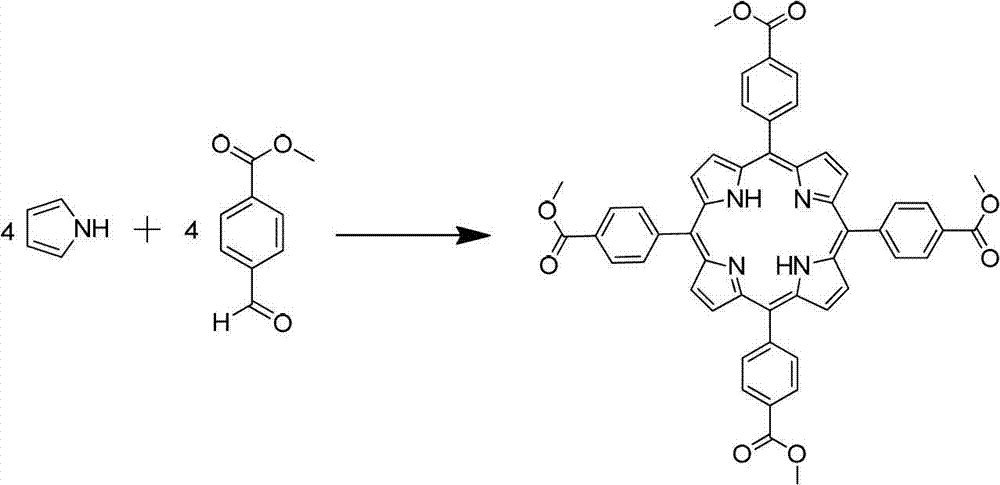
[0025] 1. Synthesis of TCPP-Me.
[0026]
[0027] In a 250mL three-neck flask equipped with a condenser, a mechanical stirrer and a constant pressure funnel, add 6.56g of methyl 4-formylbenzoate and 80mL of n-propionic acid in sequence, start stirring, then heat to reflux (132°C), pass Slowly add the mixed solution of 2.72g pyrrole and 8ml acetic anhydride to the system through the constant pressure dropping funnel (about 25 minutes to complete the dropwise addition). During the dropping process, the color of the reaction solution changes from light yellow to dark brown. After 3h, the reaction was stopped, and after cooling down to room temperature, it was placed in the refrigerator to freeze overnight.
[0028] Post-processing: filter the reaction solution, rinse the filter cake with 100ml ethanol, dry the solid to obtain 2.06g of blue-purple solid, and characterize the molecular structure by 1HNMR and MS, which is the product TCPP-Me, yield: 21% .
[0029] 2. Synthesis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com