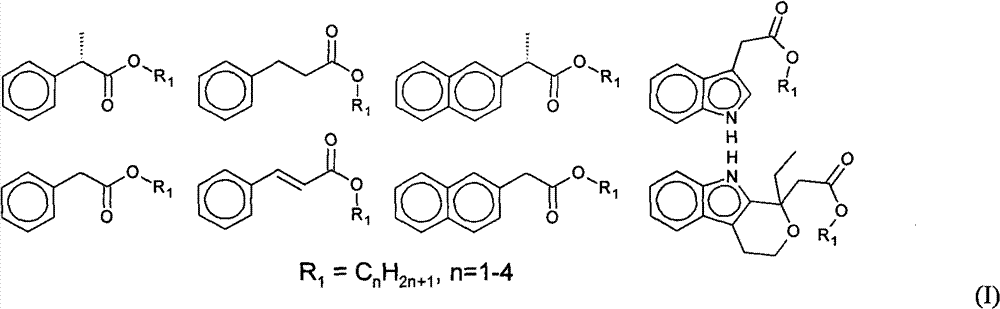
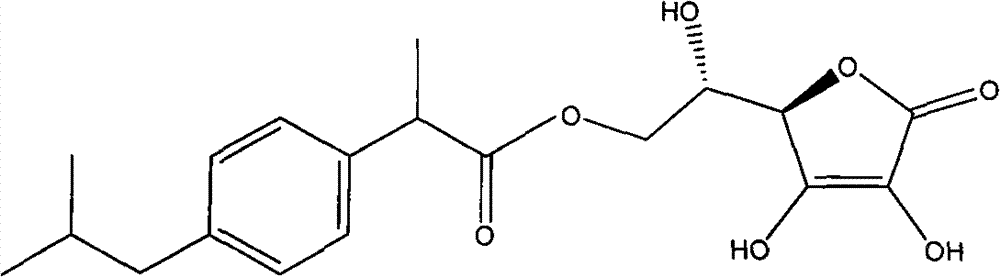
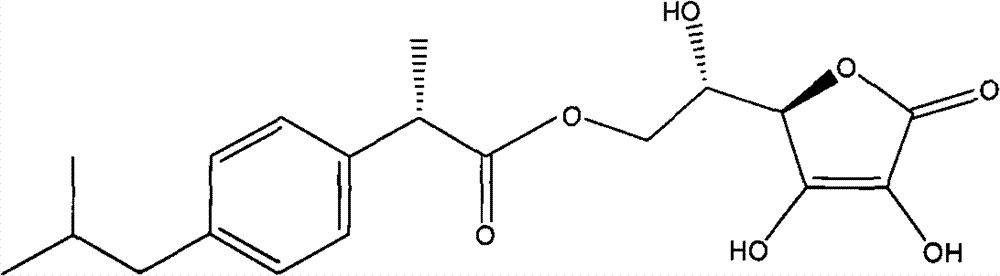
Ester exchange method synthesizing process of aryl acetic acid (propionic acid) L-ascorbic acid ester
A technology for ascorbic acid ester and synthesis process, which is applied in the field of transesterification synthesis process of arylacetic acid L-ascorbic acid ester, and can solve problems such as waste of raw materials and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, L-ascorbic acid-6-O-ibuprofen ester
[0017]
[0018] In a 250 mL three-necked flask, add 5 g of L-ascorbic acid and 100 mL of 20% (w / v) ibuprofen methyl ester-tert-butanol solution, and heat to 55° C. in a water bath. Then add 1g of Novozym435, stir the reaction, turn on the vacuum pump after 12hrs of reaction, and keep the vacuum at 0.03-0.04MPa to distill the generated methanol. After reacting for 24 hrs, a reaction mixture was obtained.
[0019] Filtrate while hot to remove unreacted reactants and enzymes to obtain a clear filtrate. The filtrate was rotary-evaporated under reduced pressure to recover tert-butanol, and the obtained mixture was weighed, and cyclohexane was added in an amount of 10%, slightly heated in a water bath at 45°C, fully stirred to dissolve, and crystallized by cooling at room temperature. Filter, wash the filter cake with cyclohexane several times, recover the filter cake, and dry the filter cake at room temperature. The o...
Embodiment 2
[0022] Embodiment 2, L-ascorbic acid-6-O-(S)-ibuprofen ester
[0023]
[0024] The method is the same as that used in Example 1, except that (S)-ibuprofen methyl ester is used instead of racemic ibuprofen methyl ester to obtain L-ascorbic acid-6-O-(S)-ibuprofen ester.
[0025] The L-ascorbic acid-6-O-(S)-ibuprofen ester is tested by liquid phase-mass spectrometry, and the purity of the product is >98%.
[0026] L-ascorbic acid is recovered and recycled in the next round of reaction.
Embodiment 3
[0027] Embodiment 3, L-ascorbic acid-6-O-ketoprofen axetil
[0028]
[0029] In a 250 mL three-necked flask, add 10 g (0.57 mol) of L-ascorbic acid and 100 mL of 25% (w / v) ketoprofen ethyl ester-tert-amyl alcohol solution, and heat to 55° C. in a water bath. Then add 1g of Novozym435, stir the reaction, turn on the vacuum pump after 12hrs of reaction, and keep the vacuum at 0.04-0.05MPa to evaporate the ethanol generated. After reacting for 24 hrs, a reaction mixture was obtained.
[0030] Filtrate while hot to remove unreacted reactants and enzymes to obtain a clear filtrate. The filtrate was rotary evaporated under reduced pressure to recover tert-amyl alcohol, the resulting mixture was weighed, and cyclohexane was added in an amount of 5%, slightly heated on a 45°C water bath, fully stirred to dissolve, and cooled to crystallize at room temperature. Filter, wash the filter cake with cyclohexane several times, recover the filter cake, and dry the filter cake at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com