Preparation and application of her2-neu antigen-positive tumor therapeutic vaccine
A her2-neu, positive technology, applied in the fields of biology and medicine, can solve the problems of not achieving satisfactory immune effect and effectively inducing immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Her2 sequence design and optimization
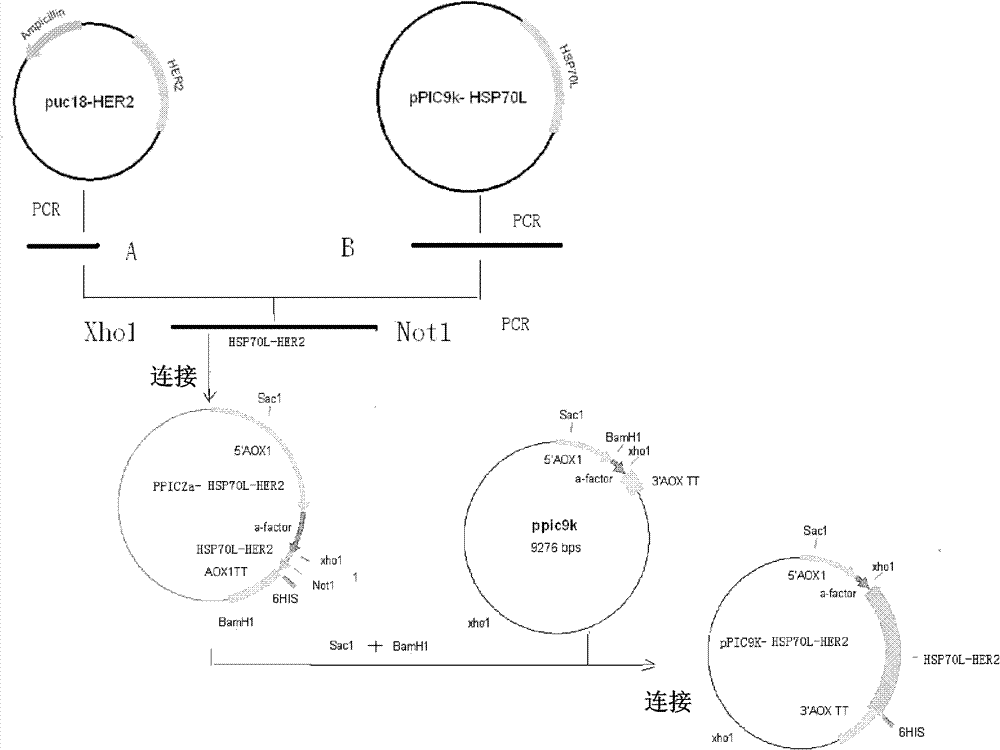
[0100] According to the Her2 nucleic acid sequence, the methanol yeast codon optimal sequence SEQ ID NO:1 was designed and synthesized, and the Not I restriction site was added at the 3'end. The sequence was ligated to the T vector pUC18 (purchased from Invitrogen), denoted as puc18-HER2 . The amino acid sequence of the Her2 / neu extracellular region element encoded by the optimized nucleotide sequence (SEQ ID NO: 1) is shown in SEQ ID NO: 2, with a length of 115, corresponding to positions 342-456.
Embodiment 2
[0102] Construction of recombinant expression plasmid
[0103] 1.Her2 342-456 And Hsp70L1 DNA fragments:
[0104] Use plasmid puc18-HER2 as template, and M13R and HSP-HER2-F as primers to obtain the encoding Her2 for constructing the fusion protein 342-456 DNA fragment (PCR product A, SEQ ID NO: 1).
[0105] The sequence of the 5'end oligonucleotide primer M13R used in the PCR reaction is:
[0106] 5'-CAGGAAACAGCTATGACC-3', (SEQ ID NO: 3).
[0107] The sequence of the 3'end oligonucleotide primer HSP-HER2-F is:
[0108] 5'-CTCTATTGAGATAGCATCTTGTTACGGTTTGGGTATG-3' (SEQ ID NO: 4).
[0109] At the same time, collect HeLa cells (ATCC Number: CCL-2) heat-induced at 41°C for 1 h, and use the cell total RNA extraction reagent Trizol (Invitrogen) to prepare total cell RNA, and use AMV reverse transcriptase (Promega) to synthesize the first Chain, using it as a template, using the following sequence of α-factor and HSP-HER2-R as PCR oligonucleotide primers to amplify to obtain a DNA fragment enco...
Embodiment 3
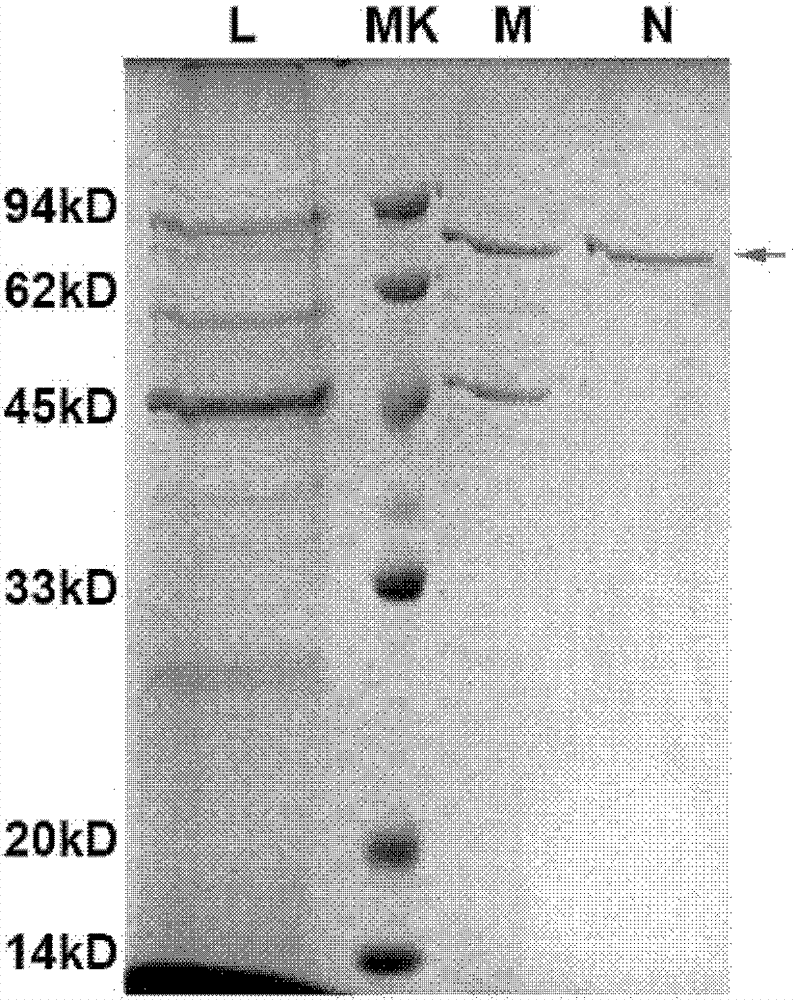
[0120] Highly expressed Hsp70L1-Her2 342-456 Construction of engineering bacteria and identification of positive yeast transformants
[0121] Kit (Qiagen Company) for mass extraction of pPIC9k-Hsp70L1-Her2 342-456 The plasmid was linearized with SacI endonuclease and electrotransformed into GS115 competent yeast cells under the transformation conditions of 2.0kV, 25μF, and 200Ω to obtain yeast transformants.
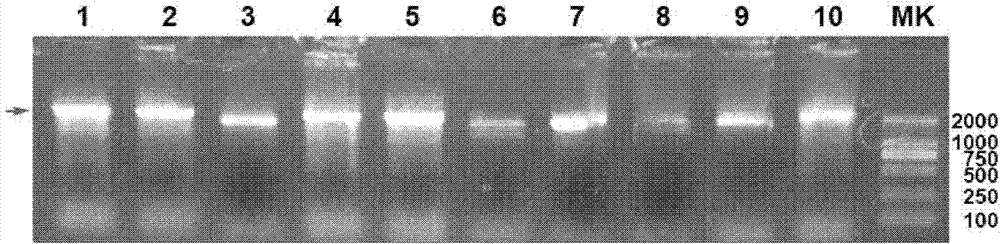
[0122] The single clones on the MD plate were respectively inoculated into 3ml YPD medium, cultured at 30°C for 24 hours, and then the yeast genomic DNA was extracted, using α-factor primer (SEQ ID NO: 4) and 3'-aox primer (3'-AOX). : 5'-GCAAATGGCATTCTGACATCC-3', (SEQ ID NO: 7)), the yeast transformants were identified by PCR. Positive recombinants were identified by electrophoresis.
[0123] The results showed that for the positive yeast transformants (denoted as pPIC9k-Hsp70L1-Her2 342-456 Yeast), a PCR band of about 2.1k was obtained ( figure 2 ), consistent with the predi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com