Quality control method of Sijunzi (Chinese name) decoction
A quality control method, the technology of Sijunzi Decoction, is applied in the field of quality control of Sijunzi Decoction to achieve good reproducibility, simple quality standards and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The content determination method for simultaneous determination of four components is:
[0020] Medicinal materials, instruments and reagents:
[0021] Ginseng (Panax ginseng C.A.Mey.; produced in Jilin, batch number 110710), Atractylodes macrocephala (Atractylodes macrocephala Koidz.; produced in Zhejiang, batch number 110306), Poria cocos (Poria cocos (Schw.) Wolf; produced in Anhui, batch number 110513) in Sijunzi Decoction , Glycyrrhizae Radix et Rhizoma Praeparata cum Melle (Glycyrrhizae Radix et Rhizoma Praeparata cum Melle; produced in Inner Mongolia, batch number 110519) were purchased from Nanjing Haiyuan Chinese Herbal Pieces Co., Ltd.
[0022] Waters2695 high-performance liquid chromatography; Waters2589 detector; UNIQUE-S15 ultrapure water generator; BP211D electronic balance (Germany Sartorius company); KQ-500DE medical numerical control ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.).
[0023] Ginsenoside Rg1 reference substance (National Ins...
Embodiment 2
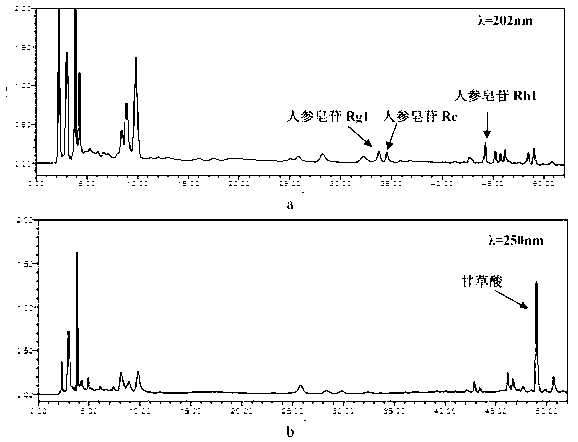
[0073] The multi-index simultaneous determination method of four components in Sijunzi Decoction is as follows:
[0074] Chromatographic conditions Chromatographic column: octadecyl bonded silica gel as filler; mobile phase: acetonitrile-0.03% formic acid solution; flow rate: 0.9mL / min; column temperature: 30°C; injection volume: 20μL. Dual wavelength detection: 202nm, 250nm. Gradient elution program: 0~14min, 19% acetonitrile; 14~15min, 20.7% acetonitrile; 15~28min, 20.7% acetonitrile; 28~33min, 29% acetonitrile; 33~47min, 41% acetonitrile; 47~52min, 41 % acetonitrile.
[0075] Preparation of Reference Substance Solution Accurately weigh appropriate amounts of ginsenoside Re, ginsenoside Rg1, ginsenoside Rb1, and ammonium glycyrrhizinate reference substance, add methanol to dissolve, and make ginsenoside Rb1, ginsenoside Re, and ginsenoside Rg1 with concentrations of 0.7995 mg respectively / mL, 0.3420mg / mL, 0.3805mg / mL mixed standard solution and ammonium glycyrrhizinate re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com