Process for the preparation of a biomass comprising plantaricin and uses thereof in medical field
A technology of plant lactobacillus and biomass, which is used in the preparation of mixtures to stimulate the barrier function of intestinal cells or human epidermal keratinocytes to achieve the effect of enhancing the barrier function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
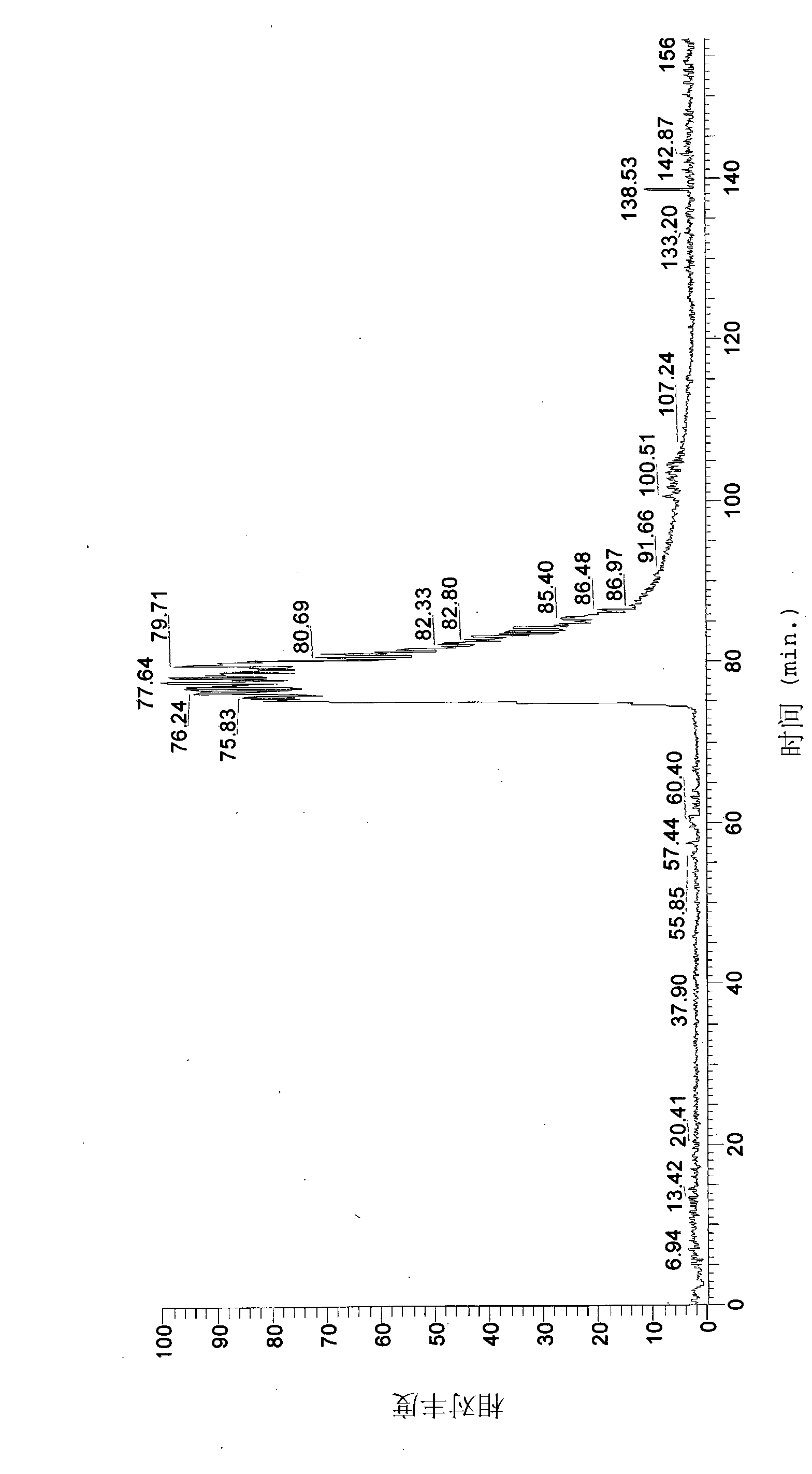
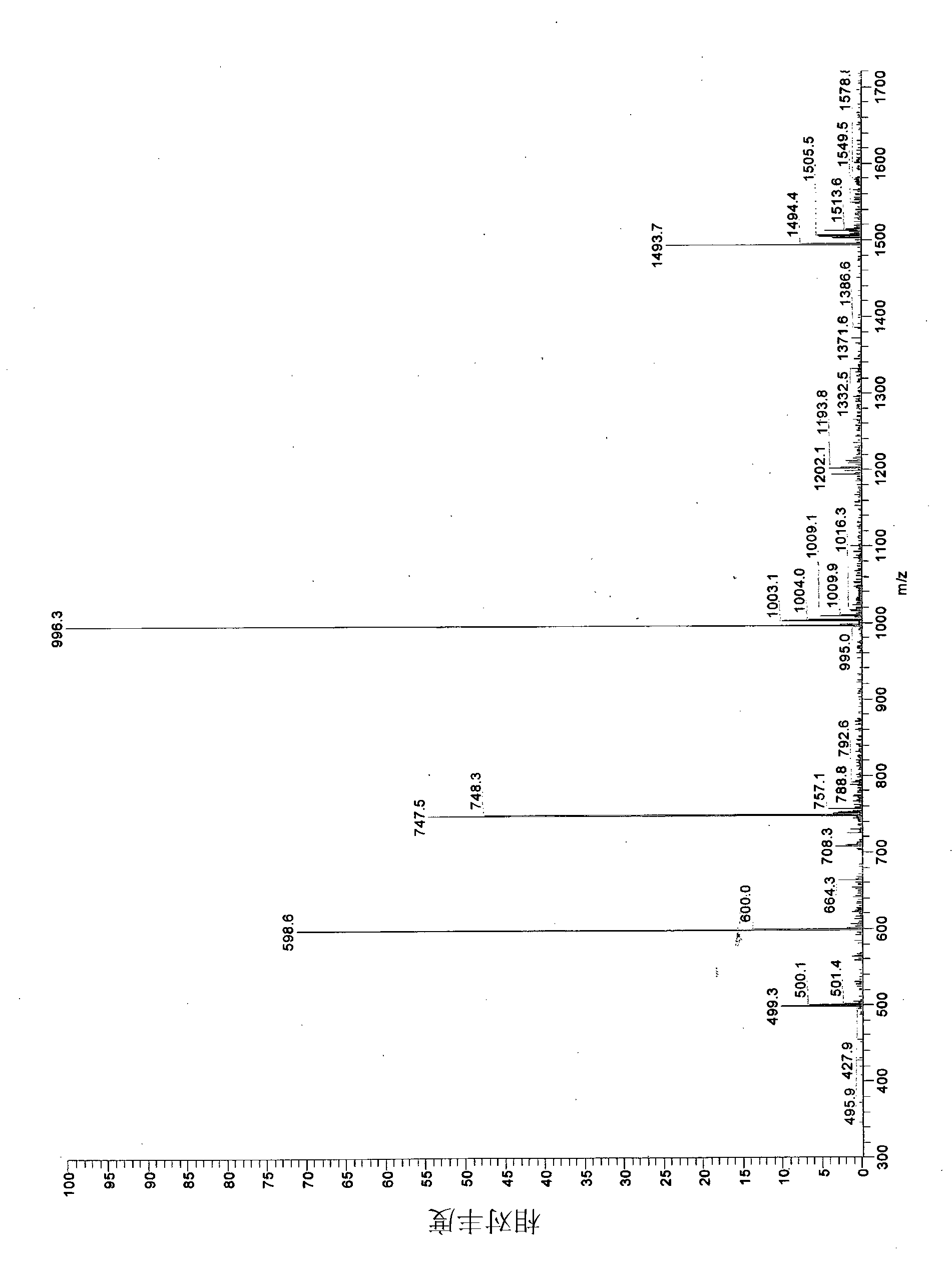
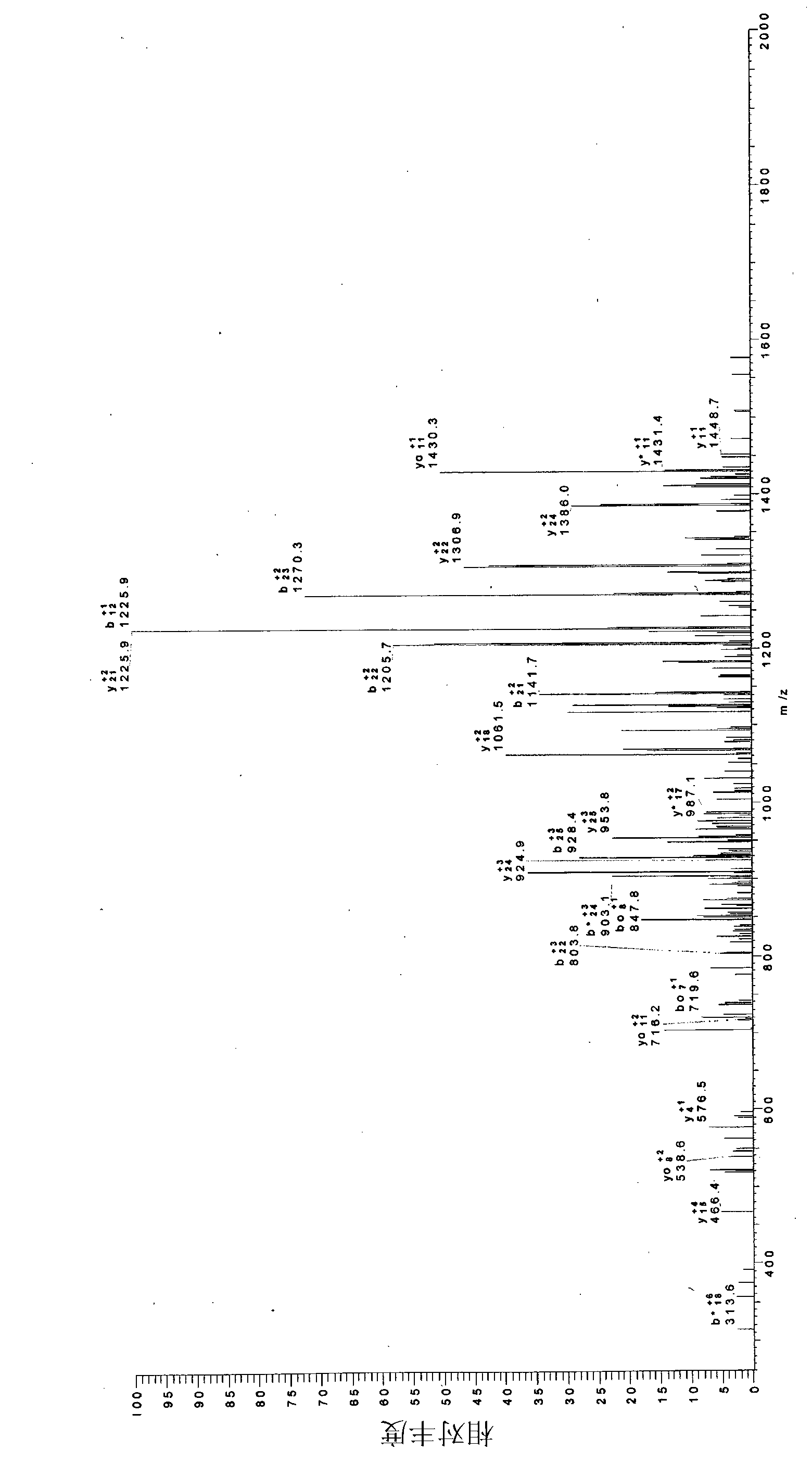
[0062] Example 1: Synthesis and purification of phytolactin-type peptide pheromones and study of their effects on epidermis and Caco-2 cells.
[0063] L. plantarum DC400 (DSM 23213) and L. rossiae DPPMA174 ( DSM), previously isolated from a "natural starter", has been propagated at 30°C for 24 hours in a modified MRS medium (mMRS) containing, in addition to the usual ingredients, 5% maltose and 10% starter water - final pH 5.6.
[0064] Cells were incubated for 24 hours, harvested by centrifugation (10.000 × g for 15 min at 4°C), washed twice with 50 mM phosphate buffer, pH 7.0, and then resuspended in water at a cell density of 9.0 log UFC / ml , inoculated (4%, each) on WFH medium under monoculture or co-culture conditions (Gobbetti, 1998.The sourdough microfora: interactions of lactic acid bacteria and sourdoughs (starter flora: lactic acid bacteria and starter interactions). Trends Food Ski Technol Vol. 9 pp. 267–274). Following the same procedure and using grape juice ...
example 2
[0103] Example 2: Research on the Effect of Biomass of the Present Invention and Plant Lactobacillus A in Wound Healing
[0104] method
[0105] Cultured human keratinocytes were incubated with phytolactin A (2.5 μg / ml) or biomass containing phytolactin A for 1 hour.
[0106] At the end of the incubation, the cells were washed and the medium was restored.
[0107] wound healing
[0108] The assay involved mechanical disruption of the continuity of the cell monolayer and was intended to assess whether the treatment favored the ability of keratinocytes to migrate beyond the damaged margin and thus "heal the injury".
[0109]To this end, keratinocyte monolayers treated as described above were cultured for an additional 24 hours after stimulation, then fixed and stained with hematoxylin / eosin staining. Images were observed using an optical microscope with a 5X objective.
[0110] For each image, detect and calculate the maximum cell migration limit and the distance or the ...
example 3
[0127] Example 3: Phytolactin A: a study of its role in maintaining and repairing skin barrier function
[0128] This study is based on the use of plantaractin A obtained from the co-cultivation of L. plantarum DC400 (DSM 23213) and L. rossiae DPPMA174 (DSM 23214), with the aim of obtaining the following objectives:
[0129] The effect of biomass on wound repair was evaluated by studying the migration and proliferation of human keratinocyte monolayers (NCTC2544) in comparison to positive controls (untreated cells) and commercially available / negative controls of known activity. Treated three times (24, 48, 72 hours) with different concentrations of test plant lactobacillus (after determination by cytotoxicity test).
[0130] Evaluate mediator modulation such as IL-8 by real-time PCR in comparison to positive controls (untreated cells) and commercially available / negative controls of known activity at the desired time and concentration , KGF (keratinocyte growth factor), TGF-β...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com