Hydrophobic catalyst with two-dimensional open hydrophobic space and preparation method thereof
A catalyst and open technology, applied in organic chemical methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve rare problems and achieve uniform dispersion and high-efficiency catalytic oxidation reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
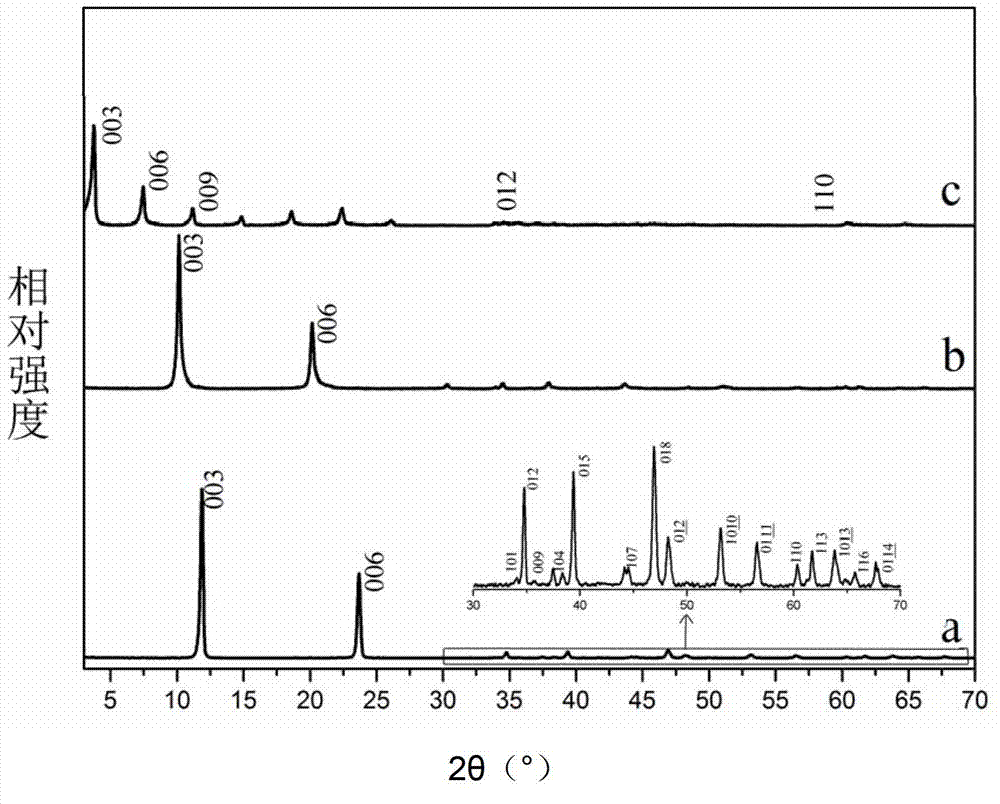
[0024] Step A: Add Zn(NO 3 ) 2 ·6H 2 O 0.007mol (about 2.0824g), Al(NO 3 ) 3 9H 2 O0.0035mol (about 1.31295g), urea 0.0245mol (about 1.4715g), prepared urea concentration 0.035mol / L, Zn 2+ Concentration 0.01mol / L, Al 3+ A solution with a concentration of 0.005mol / L. Set magnetic stirring at 105°C in an oil bath, and reflux for 27 hours. 4 times of deionized water, 3 times of anhydrous ethanol suction filtration and washing, and vacuum drying at room temperature for 12 hours to obtain Zn-Al-CO 3 LDHs.
[0025] Step B: DeCO in 600mL 2 Add Zn-Al-CO in deionized water 3 LDHs0.6000g, NaNO 3 76.4910g, HNO 3 200μL, prepared hydrotalcite 1.000g / L, NaNO 3 Concentration 1.5mol / L, HNO 3 A mixed solution with a concentration of 0.005mol / L. Under a nitrogen atmosphere, the reaction was carried out at room temperature for 48 h. Centrifuge and wash multiple times. Dry at room temperature in vacuum for 12h to get Zn-Al-NO 3 LDHs.
[0026] Step C: DeCO in 200mL 2 Add Zn-Al-...
Embodiment 2
[0030] Step A: Take a method similar to step A in Example 1 to obtain Zn-Al-CO 3 LDHs.
[0031] Step B: Obtain Zn-Al-NO by the method similar to Step B in Example 1 3 LDHs precursor.
[0032] Step C: DeCO in 200mL 2 Add Zn-Al-NO in deionized water 3 LDHs0.5g, sodium dodecylbenzenesulfonate 0.07mol / L (about 4.8787g). Set magnetic stirring at 70°C in an oil bath, and react for 48 hours. After centrifugation, washing several times, and vacuum drying at room temperature for 24 hours, Zn-Al-DBS LDHs were obtained.
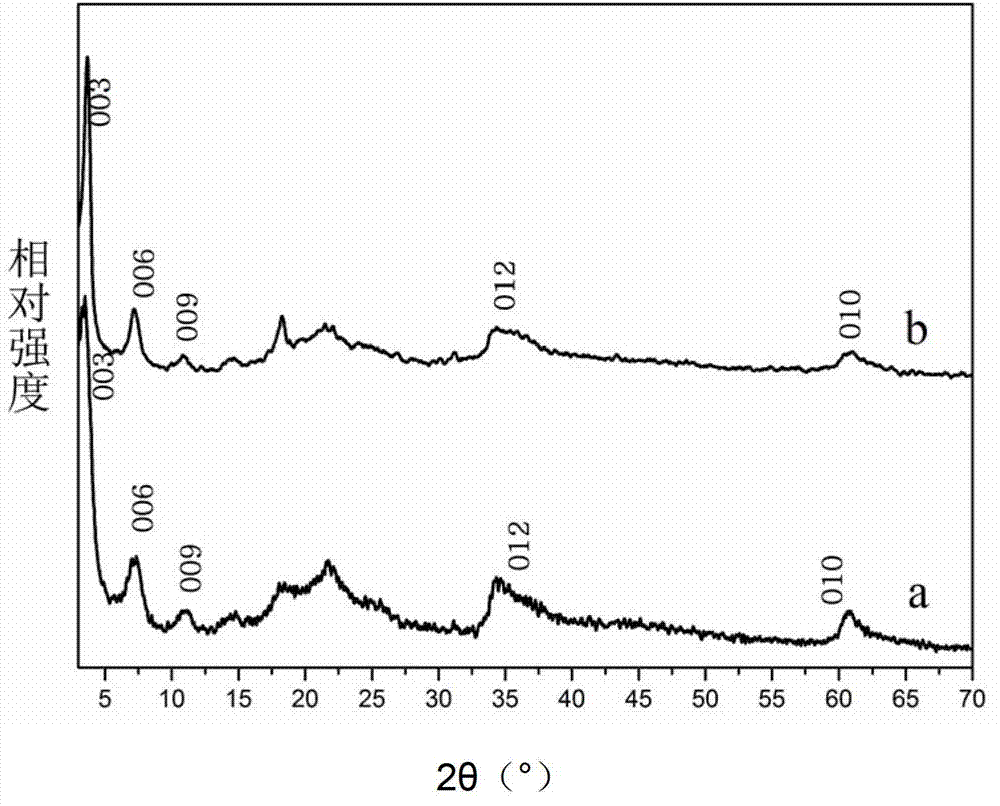
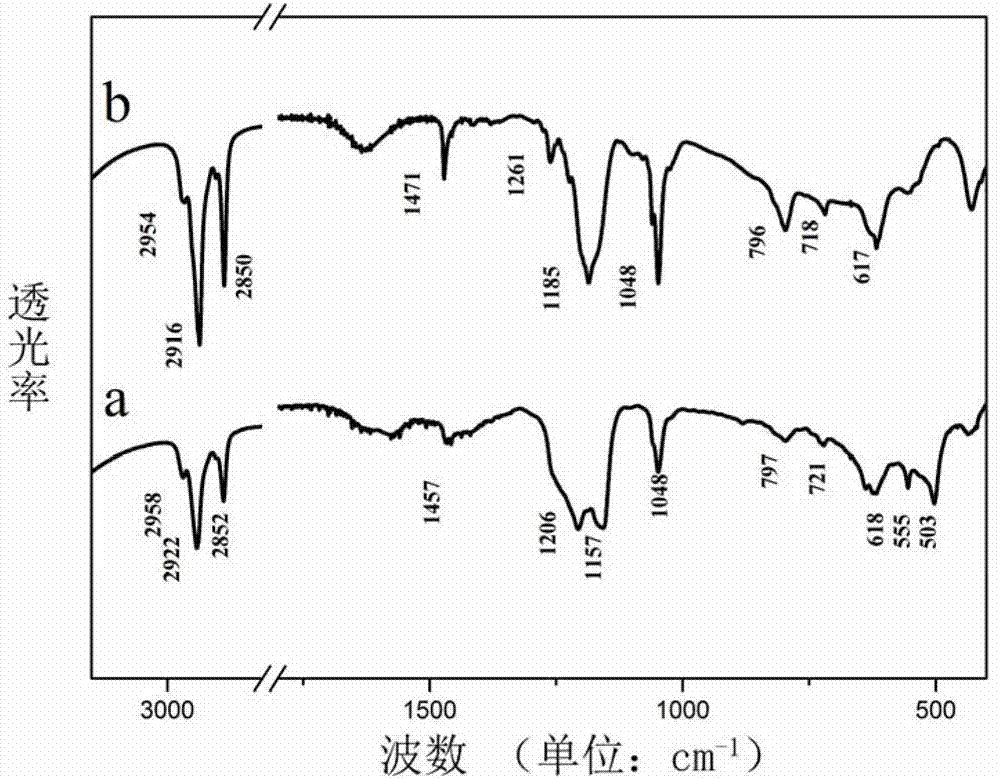
[0033] Step D: Add 0.1 g of Zn-Al-DBS LDHs to 200 mL of n-butanol, and reflux at 120° C. for 36 h. Add 0.02 g of Mn(TPP)OAc, stir and mix for 12 hours, evaporate the solvent, centrifuge at 5000 rpm, and wash with ethanol and dichloromethane. Vacuum drying, room temperature 12h, the product [Zn 0.66 Al 0.34 (OH) 2 ][(DBS 2- ) 0.17 (Mn(TPP)OAc) 0.0143 ]·0.21H 2 O.
[0034]Add 1 mg of the synthesized catalyst into an anhydrous and anaerobic reaction tube con...
Embodiment 3
[0036] Step A: Take a method similar to step A in Example 1 to obtain Zn-Al-CO 3 LDHs.
[0037] Step B: Obtain Zn-Al-NO by the method similar to Step B in Example 1 3 LDHs precursor.
[0038] Step C: DeCO in 200mL 2 Add Zn-Al-NO in deionized water 3 LDHs0.5g, sodium dodecylsulfonate (SAS) 0.05mol / L (about 2.7238g). Set magnetic stirring at 70°C in an oil bath, and react for 48 hours. Centrifuge, wash several times, and vacuum dry at room temperature for 24 hours to obtain Zn-Al-AS LDHs.
[0039] Step D: Add 0.1 g of Zn-Al-AS LDHs to 200 mL of n-butanol, and reflux at 120° C. for 36 h. Add 0.02 g of tetraphenylporphine ferric chloride Fe(TPP)Cl, stir and mix for 12 hours, evaporate the solvent, centrifuge at 5000 rpm, and wash with ethanol and dichloromethane. Vacuum drying, room temperature 12h, the product [Zn 0.62 Al 0.38 (OH) 2 ][(AS 2- ) 0.19 (Fe(TPP)Cl) 0.0107 ]·0.32H 2 o).
[0040] Add 1 mg of the synthesized catalyst into an anhydrous and anaerobic reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com