Linezolid crystal form VI and preparation method thereof
A technology of linezolid and its crystal form, which is applied in the field of linezolid crystal form VI and its preparation, can solve the problems of high power consumption, large consumption of organic solvents for crystal form A, easily damaged product purity, etc., and achieves mild process conditions, Guarantee the ideal effect of medication effect and dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 5g of linezolid crystal form A (form A disclosed in WO 2011 / 050826), add it to 75ml of water, cool down to 0°C, add 2mol / L concentrated hydrochloric acid, stir until the solid dissolves completely, and keep the internal temperature (that is, keep Solution temperature) is 0°C, dropwise add potassium hydroxide aqueous solution with a mass percentage concentration of 20%, adjust PH=7, continue to heat and stir for 30 minutes, filter, wash the solid with water until neutral, and dry Delineate under reduced pressure at 40°C Azolamide crystal form VI 4.81g.
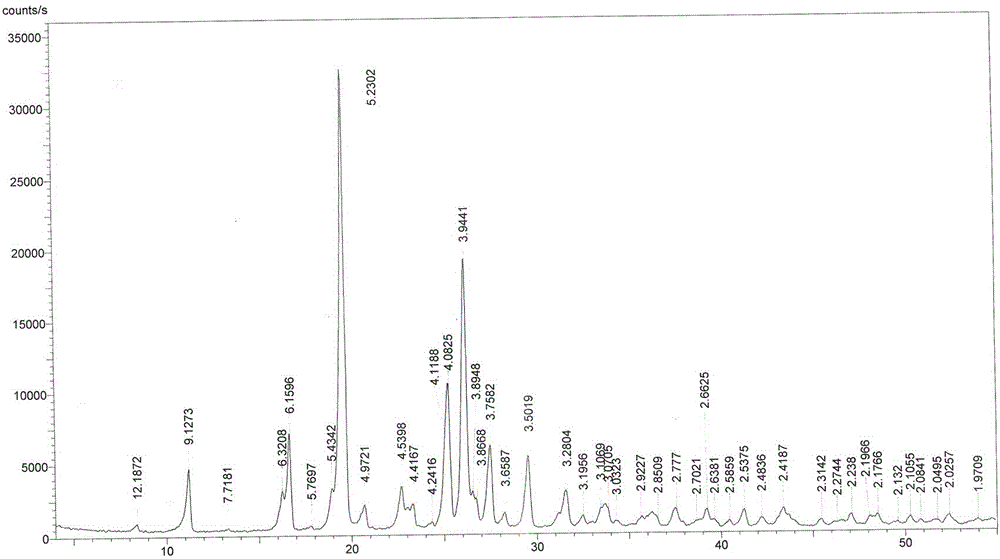
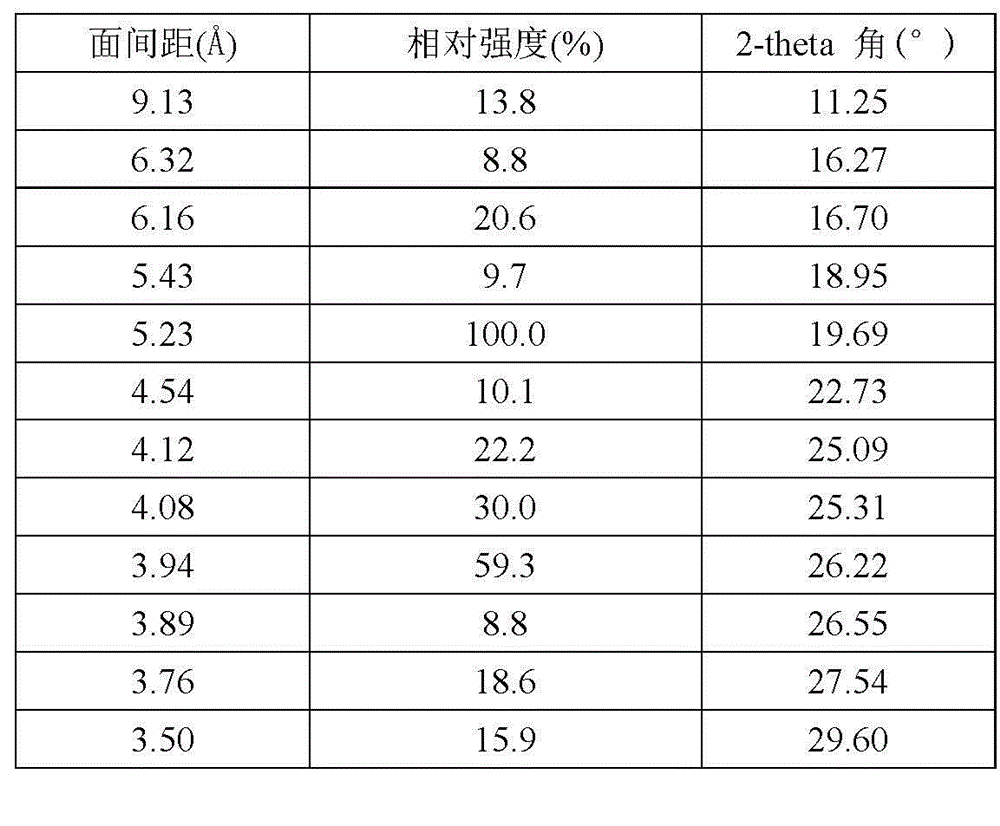
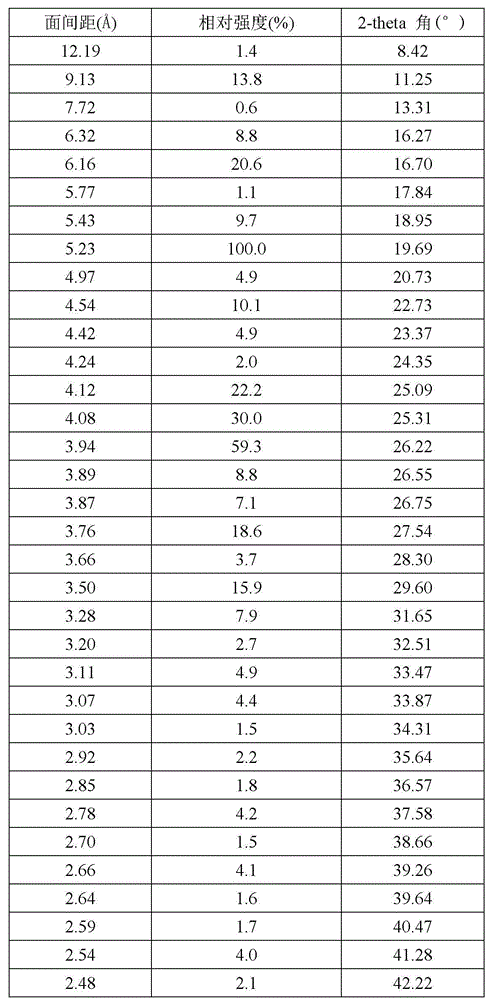
[0027] The crystal form VI of linezolid obtained in this example, the X-ray powder diffraction pattern of linezolid crystal form VI is as follows: figure 1 (The abscissa is the 2-theta angle (°), the ordinate is the relative intensity (%), and the number marked in the figure is the interplanar distance (?)). The X-ray powder diffraction pattern data are shown in the following table:
[0028] Relative Stre...
Embodiment 2
[0031] Weigh 5g of linezolid crystal form II (form II disclosed in EP1255754), add it to 25ml of water, add 3mol / L formic acid, stir until the solid dissolves completely, keep the internal temperature at 5°C, and dropwise add 32% (mass %) sodium hydroxide aqueous solution, adjust PH=12, continue to stir for 30 minutes, filter, wash the solid with water until neutral, and dry under reduced pressure at 50°C to obtain 4.77g of linezolid crystalline form VI.
Embodiment 3
[0033] Weigh 5g of linezolid crystal form III (form III disclosed in EP2100884), add it to 40ml of water, add acetic acid, stir until the solid dissolves completely, add 50% potassium carbonate aqueous solution to it dropwise at room temperature, adjust the pH=10, continue Stir for 30 minutes, filter, wash the solid with water until neutral, and dry under reduced pressure at 40°C to obtain 4.72 g of Linezolid Form VI.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com