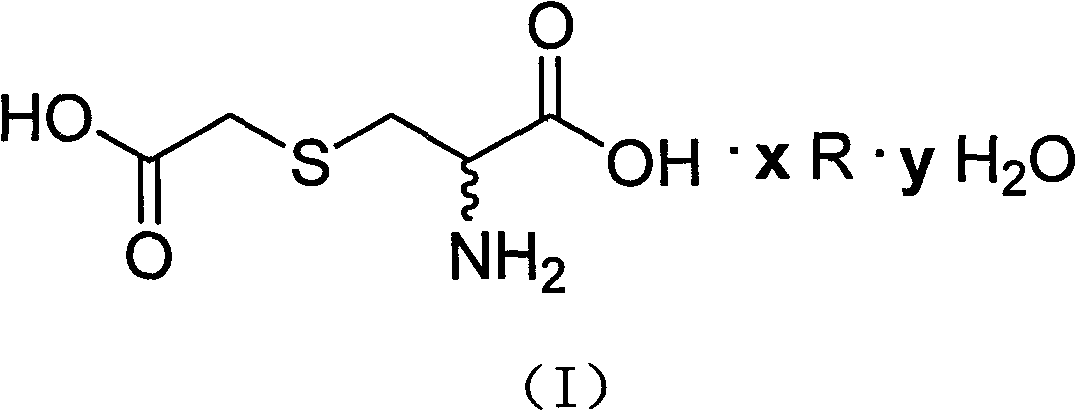
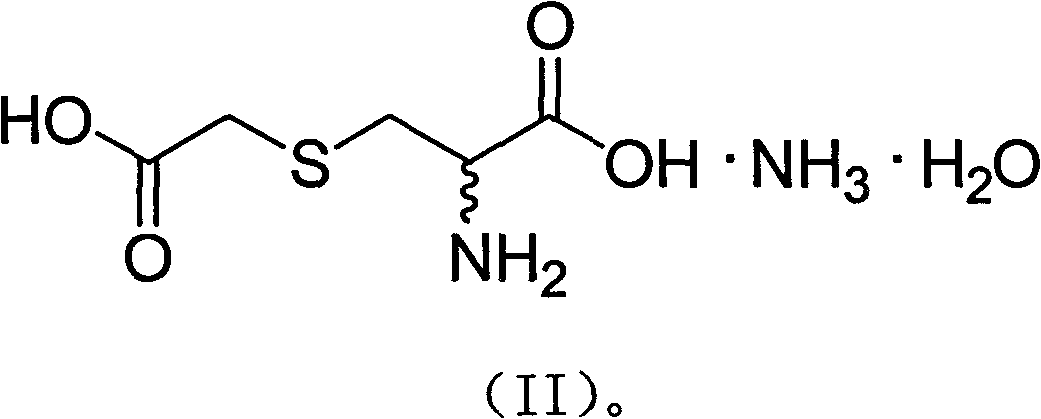
S-(carboxymethyl)-cysteine pharmaceutical compound and preparation method and usage thereof
A pharmaceutical compound, cysteine technology, applied in the preparation of organic compounds, drug combinations, chemical instruments and methods, etc., can solve problems such as damage to gastrointestinal mucosa, bleeding, ulcers and even perforation, and irritating effects on the digestive tract
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
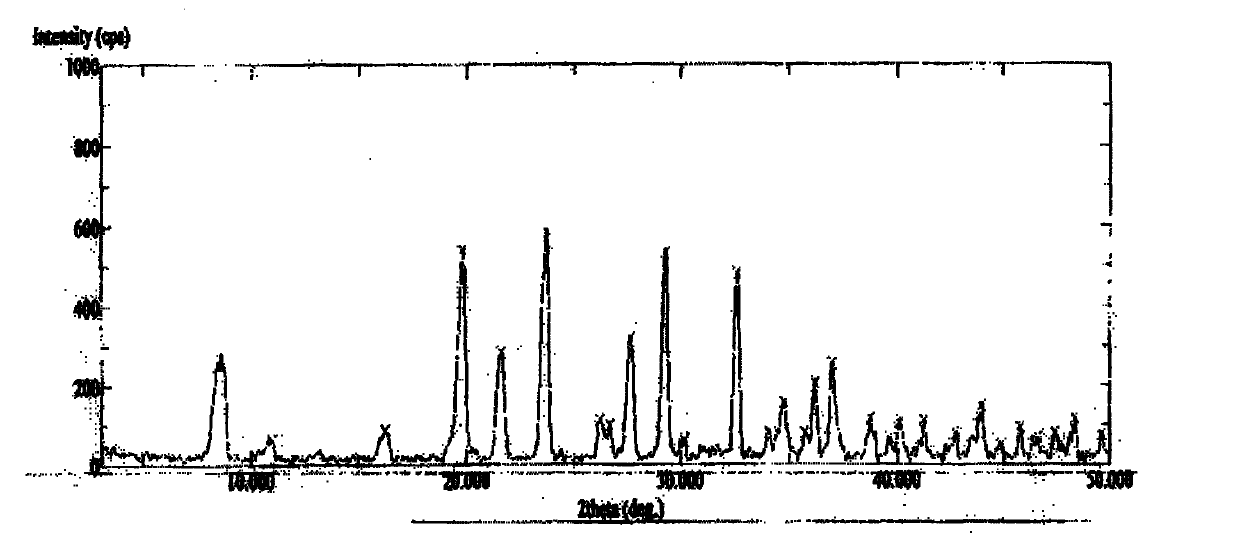
Image
Examples
Embodiment 1
[0089] Preparation of S-(carboxymethyl)-D-cysteine arginine salt tetrahydrate crystalline compound:
[0090] At room temperature, add 20g of S-(carboxymethyl)-D-cysteine, 19.5g of arginine, and 50ml of distilled water into a three-neck flask equipped with a stirring device, heat the water bath to 50°C, keep stirring to make the reaction solution After dissolving and clearing, add 100ml of n-butanol dropwise to the reaction solution. The dropping speed is slightly faster at the beginning, and slow down when white crystals are precipitated. The filter cake was washed with n-butanol, sucked dry, and dried to obtain 37.5 g of white S-(carboxymethyl)-D-cysteine arginine salt tetrahydrate crystals, with a yield of about 79.0%. The moisture content was 16.95%, and the element analysis results were C 31.12%, S 7.54%, N 16.86%, and O 37.66%.
Embodiment 2
[0092] Preparation of S-(carboxymethyl)-D-cysteine arginine salt pentahydrate crystalline compound:
[0093] At room temperature, add 25g of S-(carboxymethyl)-D-cysteine, 24.5g of arginine, and 20ml of distilled water into a three-neck flask equipped with a stirring device, heat the water bath to 50°C, keep stirring to make the reaction solution After dissolving, add dropwise the mixed solution of 50ml isopropanol and 50ml tetrahydrofuran therein. At the beginning, the drop rate is slightly faster, and when white crystals are precipitated, the drop rate is slowed down. Wash the filter cake with a small amount of the above-mentioned mixed solution, drain it, and dry it to obtain 54.8 g of white S-(carboxymethyl)-D-cysteine arginine salt pentahydrate crystals, with a yield of about 88.6%. The moisture determination result is 20.1%, and the elemental analysis results are C 29.73%, S 7.24%, N 15.86%, O 39.61%.
Embodiment 3
[0095] Preparation of S-(carboxymethyl)-L-cysteine arginine salt trihydrate crystalline compound:
[0096] At room temperature, add 20g of S-(carboxymethyl)-L-cysteine, 19.5g of arginine, and 50ml of distilled water into a three-neck flask equipped with a stirring device, heat the water bath to 50°C, keep stirring to make the reaction solution After dissolving and clearing, add 300ml of ethanol dropwise to the reaction liquid. The dropping speed is slightly faster at the beginning, and slow down when white crystals are precipitated. After the ethanol is added dropwise, grow crystals at 20°C, filter with suction, and wash the filter cake with a small amount of ethanol. , drained, and dried to obtain 42.7 g of white S-(carboxymethyl)-L-cysteine arginine salt trihydrate crystals, with a yield of about 93.9%. The moisture content was 13.7%, and the element analysis results were C 32.42%, S 7.96%, N 17.23%, and O 35.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com