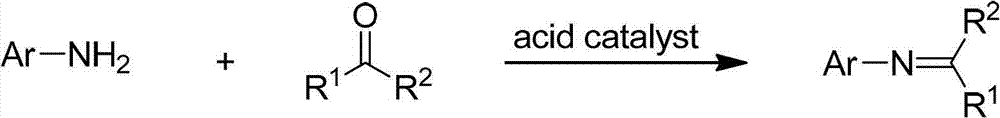Method for synthesizing N-aryl ketoimine by acidic catalytic dehydration
A technology of aryl ketimine and acid catalysis, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of cumbersome post-processing, low yield, unsuitable for large-scale preparation, etc., and achieve product The effect of high yield, high conversion rate of raw materials and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of immobilized perchloric acid
[0025] Stir 25.0 g of silica gel (200-400 mesh) and 2.0 g of 70% perchloric acid aqueous solution in 50 mL of ether for 1 hour, concentrate and dry at 100°C for 24 hours under vacuum to obtain 26.2 g of white powder. The acid equivalent is: 0.53±0.02 meq / g.
Embodiment 2
[0026] Embodiment 2: the preparation of immobilized sulfuric acid
[0027] Within 30 minutes, drop 19.4 grams of chlorosulfonic acid into 50.0 grams of dry silica gel (200-400 mesh), shake for 30 minutes after dropping, wash with water and dry at 100°C for 24 hours under vacuum to obtain 61.5 grams of white powder . The acid equivalent is: 2.51±0.02 meq / g.
Embodiment 3
[0028] Embodiment 3: the preparation of immobilized benzenesulfonic acid
[0029] In a 500mL reaction bottle, add 5g of activated carbon, 43g of 4-diazobenzenesulfonic acid aqueous solution and 100mL of ethanol, place the reaction system in an ice-water bath and stir, and keep the temperature in the bottle at 0-5°C within 30 minutes. 250 mL of 50% H 3 PO 2 The aqueous solution continued to stir for 1 hour after dripping, filtered, washed with water, washed with acetone, and dried at 100° C. under vacuum for 24 hours to obtain 23.0 grams of black powder. The acid equivalent is: 4.99±0.02 meq / g.
[0030] In the following examples, unless there is a special statement, the order of adding the reaction materials can be freely combined among the materials. In the embodiment, 2-methyl-6-ethylaniline (content 97%) and methoxyacetone (content 96%) are used as raw materials to prepare the product 2-methyl-6-ethyl-N-(2-methyl Oxygen-1-methylethylene)aniline (imine for short) is mainl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com