Preparation method of ion exchange resin
A technology of ion exchange resin and divinylbenzene, which is applied in the field of uranium mining and smelting, can solve the problems of difficult leaching and high price, and achieve the effects of reducing reagent consumption and improving leaching performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
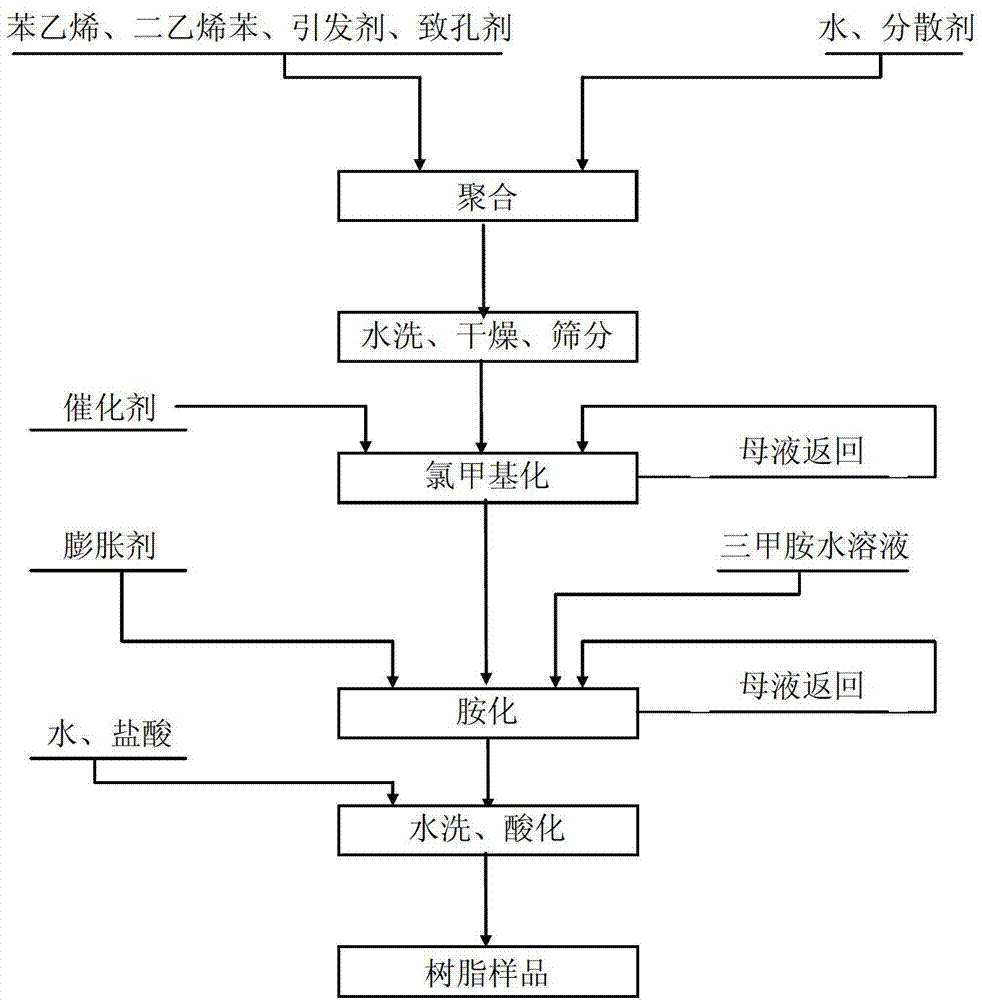
[0026] Such as figure 1 Shown, the preparation method of a kind of ion exchange resin of the present invention comprises the steps:
[0027] (1) Suspension polymerization reaction
[0028] Prepare dispersant solution: add gelatin to 1L of water, stir to obtain gelatin solution, wherein the amount of gelatin added is 8g / L; then add 16ml of methylene blue aqueous solution with a mass percentage concentration of 0.1% to each liter of gelatin solution.
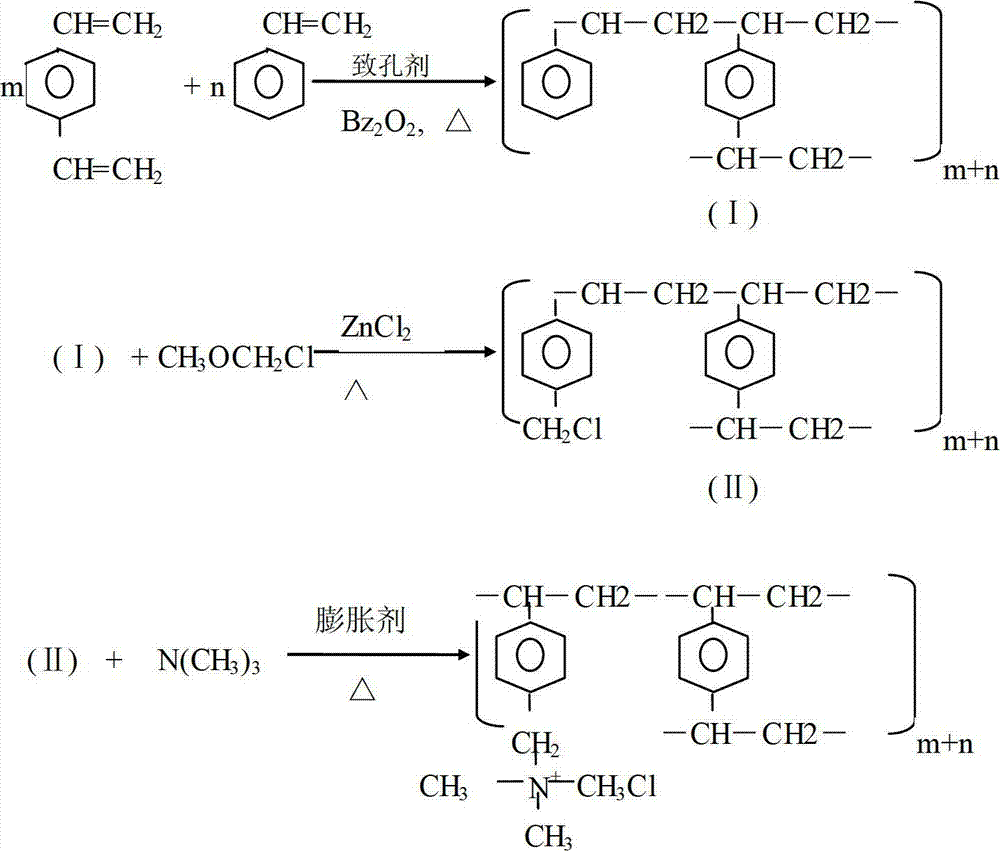
[0029] Prepare the organic phase: mix divinylbenzene (DVB) and styrene (ST) evenly, wherein the mass percentage of divinylbenzene is 25%, and the mass percentage of styrene is 75%; then add divinylbenzene and styrene to the mixture Add dibenzoyl peroxide (BPO) and porogen to the mixture, the added quality of dibenzoyl peroxide is 0.8% of the total mass of divinylbenzene and styrene mixture, the added quality of porogen is divinylbenzene and 60% of the total mass of styrene mixture;
[0030] The porogen includes component A and ...
Embodiment 2
[0042] Such as figure 1 Shown, the preparation method of a kind of ion exchange resin of the present invention comprises the steps:
[0043] (1) Suspension polymerization reaction
[0044] Prepare dispersant solution: add gelatin to 1L of water, stir to obtain gelatin solution, wherein the amount of gelatin added is 5g / L; then add 10ml of 0.1% methylene blue aqueous solution to each liter of gelatin solution.
[0045] Prepare the organic phase: mix divinylbenzene (DVB) and styrene (ST) evenly, wherein the mass percentage of divinylbenzene is 15%, and the mass percentage of styrene is 85%; then add divinylbenzene and styrene to the mixture Dibenzoyl peroxide (BPO) and porogen are added to the mixture, the added mass of dibenzoyl peroxide is 0.5% of the total mass of divinylbenzene and styrene mixture, and the added mass of porogen is divinylbenzene and 40% of the total mass of styrene mixture;
[0046] The porogen includes component A and component B, component A is liquid p...
Embodiment 3
[0056] Such as figure 1 Shown, the preparation method of a kind of ion exchange resin of the present invention comprises the steps:
[0057] (1) Suspension polymerization reaction
[0058] Prepare dispersant solution: add gelatin to 1L of water, stir to obtain gelatin solution, wherein the amount of gelatin added is 10g / L; then add 20ml of 0.1% methylene blue aqueous solution to each liter of gelatin solution.
[0059] Prepare the organic phase: mix divinylbenzene (DVB) and styrene (ST) evenly, wherein the mass percentage of divinylbenzene is 20%, and the mass percentage of styrene is 80%; then add divinylbenzene and styrene to the mixture Dibenzoyl peroxide (BPO) and porogen are added to the mixture, the added mass of dibenzoyl peroxide is 1% of the total mass of divinylbenzene and styrene mixture, and the added mass of porogen is divinylbenzene and 70% of the total mass of styrene mixture;
[0060] The porogen includes component A and component B, component A is liquid pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com