Method for preparing decoquinate intermediate 2-oxethyl-4-nitrophenol potassium
A technology of diethoxynitrobenzene and potassium nitrophenolate, which is applied in the preparation of the intermediate 2-ethoxy-4-nitrophenolate of the anticoccidial drug decoquinate, the intermediate of decoquinate In the field of preparation, it can solve the problems of low cost, long reaction time, waste of raw materials, etc., and achieve the effects of mild reaction conditions, reduced reaction time, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of decoquinate intermediate 2-ethoxy-4-nitrophenate potassium, the steps are as follows:
[0033] Add 36g of 3,4-diethoxynitrobenzene, 32.6g of potassium hydroxide, 300ml of ethylene glycol, and benzyltributylammonium chloride into a 1000ml three-necked flask equipped with a reflux condenser, a thermometer and a magnetic stirring device 0.72g, under the condition of reaction temperature of 110°C, stir and reflux for 10h, monitor the reaction in liquid phase to complete the reaction, cool down to 0-5°C to crystallize, let the crystal grow for 3 hours, filter, and wash the precipitate twice with ethyl acetate , 100ml each time, dried at 80°C to obtain 33.6g of potassium 2-ethoxy-4-nitrophenate, with a molar yield of 89.1%;
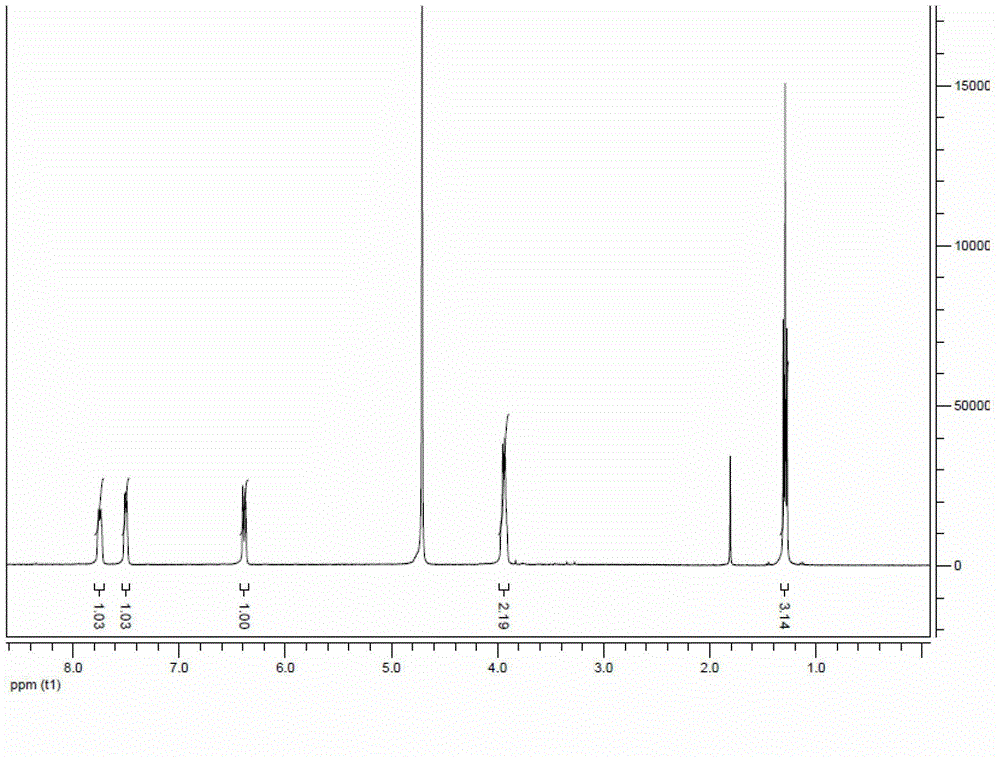
[0034] According to the structure confirmation of nuclear magnetic spectrum, the obtained product is potassium 2-ethoxy-4-nitrophenolate. The NMR results are as figure 1 Shown as: 1 H-NMR (CDCl 3 )δ7.74(d,1H,Ar-H),7.50...
Embodiment 2
[0036] A kind of preparation method of decoquinate intermediate 2-ethoxy-4-nitrophenate potassium, the steps are as follows:
[0037] Add 42.2g of 3,4-diethoxynitrobenzene, 38.2g of potassium hydroxide, 57.3ml of water, 352ml of ethylene glycol monoethyl ether and benzyl Tributyl ammonium chloride 3.4g, at the reaction temperature of 108°C, stirred and refluxed for 10h, the liquid phase monitored the reaction was completed, cooled to 0-5°C to crystallize, stood for 3 hours to grow crystals, filtered, and precipitated Wash twice with ethyl acetate, 100ml each time, and dry at 80°C to obtain 39.8g of potassium 2-ethoxy-4-nitrophenolate, with a molar yield of 90.0%;
[0038] According to the structure confirmation of nuclear magnetic spectrum, the obtained product is potassium 2-ethoxy-4-nitrophenolate. The NMR results are: 1 H-NMR (CDCl 3 )δ7.74(d,1H,Ar-H),7.50(s,1H,Ar-H),6.38(d,1H,Ar-H),3.94(q,2H,-OCH 2 CH 3 ),1.29(t,3H,-OCH 2 CH 3 ).
Embodiment 3
[0040] A kind of preparation method of decoquinate intermediate 2-ethoxy-4-nitrophenate potassium, the steps are as follows:
[0041] Add 36g of 3,4-diethoxynitrobenzene, 32.6g of potassium hydroxide, 293.4ml of water, 300ml of ethylene glycol dimethyl ether and benzyl Tributyl ammonium chloride 3.6g, under the condition of reaction temperature of 80°C, stirred and refluxed for 10h, the liquid phase monitored the completion of the reaction, cooled to 0-5°C to crystallize, stood for 3 hours to grow crystals, filtered, and precipitated Wash twice with ethyl acetate, 100ml each time, and dry at 80°C to obtain 33.4g of potassium 2-ethoxy-4-nitrophenolate, with a molar yield of 88.5%;
[0042] According to the structure confirmation of nuclear magnetic spectrum, the obtained product is potassium 2-ethoxy-4-nitrophenolate. The NMR results are: 1 H-NMR (CDCl 3 )δ7.74(d,1H,Ar-H),7.50(s,1H,Ar-H),6.38(d,1H,Ar-H),3.94(q,2H,-OCH 2 CH 3),1.29(t,3H,-OCH 2 CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com