Hyaluronan and biodegradable high polymer modified material and preparation method
A technology of polymer material and hyaluronic acid, which is applied in the field of hyaluronic acid and biodegradable polymer modified materials and preparation, can solve the problem that hyaluronic acid cannot achieve the therapeutic effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
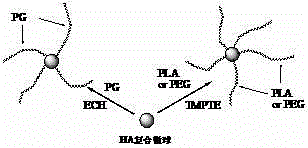
[0049] Example 1: Composite cross-linking of hyaluronic acid and sodium alginate (the first type of polymer)
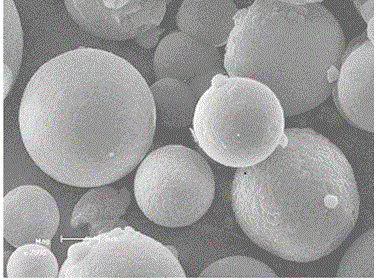
[0050] 4g of hyaluronic acid and 1g of sodium alginate were dissolved in 20mL of 2% (mass fraction) tetrabutylammonium hydroxide aqueous solution. 4%), stirred and reacted at 25°C for 20~24h, adjusted the pH=7-7.5 with 2mol / L hydrochloric acid after the reaction, washed the gel with 200mL of 75% ethanol aqueous solution and dehydrated the gel, at 50°C , dried under a vacuum of 0.08-0.09MPa for 4-6h; then the gel was swelled in 200mL pH=7 phosphate buffer for 72-96h, and when the swelling equilibrium was reached, it was taken out and soaked in 15% chloride Calcium aqueous solution, take it out after 24-36 hours, you can get hyaluronic acid-sodium alginate gel with higher hardness. It has been tested that the maximum destructive pressure that the gel can withstand is 30N, while the maximum destructive pressure that the cross-linked hyaluronic acid gel with the same cro...
Embodiment 2
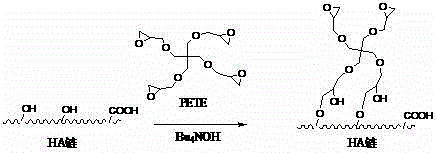
[0051] Example 2: Hyaluronic acid grafted polylactic acid (PLA) (hydroxyl grafted)
[0052] Dissolve 2g of hyaluronic acid in 50mL of 2% tetrabutylammonium hydroxide aqueous solution; then dissolve 30g of polylactic acid in 150mL of dichloromethane, and then add 0.6g of cross-linking agent pentaerythritol tetraglycidyl ether to the solution (PETE); mix the aqueous solution and dichloromethane solution under stirring, react at 25°C for 12-14h, after the reaction is completed, use 2mol / L hydrochloric acid to adjust the pH to 7~7.5, and then under the vacuum of 0.09MPa The dichloromethane was distilled off, and the reaction product was washed with 400 mL of ethanol to dehydrate and remove tetrabutylammonium chloride impurities to obtain an amphiphilic graft polymer.
[0053]
Embodiment 3
[0054] Example 3: Hyaluronic acid grafted polylactic acid (PLA) (carboxyl grafted)
[0055] Dissolve 2g of hyaluronic acid in 50mL of tetrabutylammonium chloride aqueous solution with a mass fraction of 2%, adjust the pH to 4.0~5.0 with 1mol / L hydrochloric acid; then dissolve 10g of polylactic acid in 50mL of dichloromethane, add Diamine 0.2g is used as cross-linking agent; after mixing dichloromethane solution and hyaluronic acid aqueous solution, add 0.4g dicyclohexylcarbodiimide (DCC) as carboxyl activator and 0.4g N-hydroxysuccinimide Amine is used as a co-activator, the temperature is raised to 40°C, and the reaction is carried out for 2~3h. After the reaction is completed, the pH is adjusted to 7~7.5 with 1% NaOH solution, and the dichloromethane is sucked dry at 25°C under a vacuum of 0.09MPa. The reaction product was then washed with 400 mL of ethanol to dehydrate and remove impurities to obtain a grafted polymer.
[0056]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com