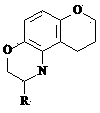
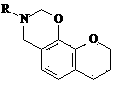
Neooxazine compounds and their uses
A compound, oxazine technology, applied in the field of medicine, can solve the problems of poor stability and low content of natural flavonoids, and achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 3-Benzyl-2,3,4,6,7,8-hexahydrochromeno[6,7-e][1,3]oxazine
[0027] Dissolve 5 mmol of 7-hydroxychroman in 2 mL of 95% ethanol, add 1.2 mL of 37% formaldehyde aqueous solution, stir for a few minutes, add dropwise 5 mmol of 7-hydroxychroman dissolved in 2-3 mL of 95% ethanol under ice cooling Benzylamine, stirred overnight at room temperature, concentrated and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 30:1) to obtain 3-benzyl-2,3,4,6,7,8-hexahydrochromene [6,7-e][1,3]oxazine as a white solid, yield: 31%. MS m / z (M) 281.35. 1 HNMR (CDCl 3 ):δ2.04(2H,m),2.55(2H,m),3.62(4H,s),3.94(2H,m),5.01(2H,s),6.11(1H,s),6.70(1H, s), 7.10(5H,m).
Embodiment 2
[0028] Example 2: 3-Phenyl-2,3,4,6,7,8-Hexahydrochromeno[6,7-e][1,3]oxazine
[0029] According to the method of Example 1, the white solid 3-phenyl-2,3,4,6,7,8-hexahydrochromene was obtained by reacting 7-hydroxychroman with aqueous formaldehyde and aniline and separated by column chromatography ,7-e][1,3]oxazine, yield: 42%. MS m / z (M) 267.32. 1 HNMR (CDCl 3 ): δ2.04(2H,m),2.55(2H,m), 3.94(2H,m), 4.61(2H,s), 6.00(2H,s), 6.11(1H,s), 6.60(3H, m), 6.70(1H,s), 7.08(2H,m).
Embodiment 3
[0030] Example 3: 3-(2-methylphenyl)-2,3,4,6,7,8-hexahydrochromeno[6,7-e][1,3]oxazine
[0031] According to the method of Example 1, the white solid 3-(2-methylphenyl)-2,3,4,6,7 was obtained by reacting 7-hydroxychroman with formaldehyde aqueous solution and 2-methylaniline and separated by column chromatography ,8-Hexahydrochromeno[6,7-e][1,3]oxazine, yield: 37%. MS m / z (M) 281.35. 1 HNMR (CDCl 3 ): δ2.04(2H,m), 2.35(3H,s), 2.55(2H,m),3.94(2H,m),4.61(2H,s),6.00(2H,s),6.11(1H, s), 6.47(2H,m), 6.70(1H,s), 6.88(2H,m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com