Method for testing forming capability of anion hydrogen bond of imidazole type ionic liquid
The technology of an ionic liquid and a measurement method is applied in the field of determination of the hydrogen bond formation ability of imidazole-type ionic liquid anions, which can solve the problems of inconvenient operation, lengthy measurement time, and complicated measurement procedures, and achieve less measurement equipment, shorter measurement time, and better measurement efficiency. The effect of simple procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
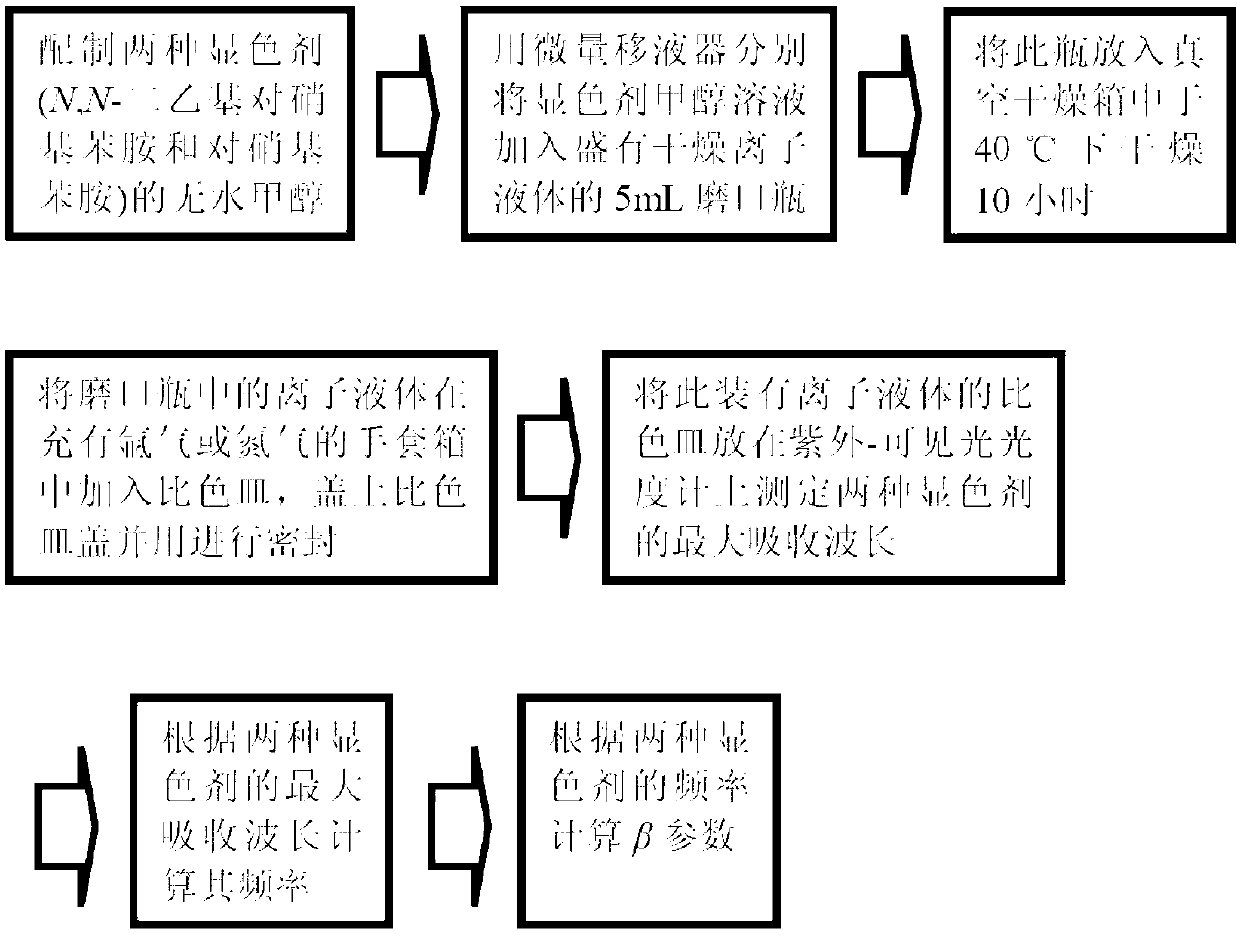
[0024] 1-Butyl-3-methylimidazole formate [C 4 mim][HCOO], 1-butyl-3-methylimidazolium butyrate [C 4 mim][CH 3 CH 2 CH 2 COO] ionic liquid b Determination of parameter values: Weigh 0.2-0.3 g of dry imidazole-type ionic liquid in NMR tubes respectively, and then weigh a given amount of deuterated dimethyl sulfoxide ([D 6 ]DMSO) to make 1.0mol / kg ionic liquid solution, and then measure the proton nuclear magnetic chemical shift of imidazole-type ionic liquid in NMR. Ionic liquid [C 4 mim][HCOO], [C 4 mim][CH 3 CH 2 CH 2 Chemical shift of 2-position hydrogen on COO] imidazole ring δ 2 They are 9.989 ppm and 10.311 ppm respectively. Will δ 2 Substitute into formula 1 to get the two kinds of ionic liquid b The parameter values are 1.002 and 1.140, respectively.
[0025] [C 4 The NMR spectrum of mim][HCOO] is as follows Figure 4 As shown, [C 4 mim][CH 3 CH 2 CH 2 The NMR spectrum of COO] such as Figure 5 shown.
Embodiment 2
[0027] 1-allyl-3-methylimidazolium propionate [Amim][CH 3 CH2 COO], 1-allyl-3-methylimidazolium butyrate [Amim][CH 3 CH 2 CH 2 COO] ionic liquid b Determination of parameter values: Weigh 0.2-0.3 g of dry imidazole-type ionic liquid in NMR tubes respectively, and then weigh a given amount of deuterated dimethyl sulfoxide ([D 6 ]DMSO) to make 1.0mol / kg ionic liquid solution, and then measure the proton nuclear magnetic chemical shift of imidazole-type ionic liquid in NMR. Ionic liquid[Amim][CH 3 CH 2 COO], [Amim][CH 3 CH 2 CH 2 Chemical shift of 2-position hydrogen on COO] imidazole ring δ 2 They are 10.385 ppm and 10.389 ppm respectively. Will δ 2 Substitute into formula 1 to get the two kinds of ionic liquid b The parameter values are 1.162 and 1.168, respectively. [Amim][CH 3 CH 2 The NMR spectrum of COO] such as Image 6 Shown, [Amim][CH 3 CH 2 CH 2 The NMR spectrum of COO] such as Figure 7 shown.
Embodiment 3
[0029] 1-Butyl-3-methylimidazole lactate [C 4 mim][CH 3 CHOHCOO], 1-allyl-3-methylimidazole lactate [Amim][CH 3 CHOHCOO] ionic liquid b Determination of parameter values: Weigh 0.2-0.3 g of dry imidazole-type ionic liquid in NMR tubes respectively, and then weigh a given amount of deuterated dimethyl sulfoxide ([D 6 ]DMSO) to make 1.0mol / kg ionic liquid solution, and then measure the proton nuclear magnetic chemical shift of imidazole-type ionic liquid in NMR. Ionic liquid [C 4 mim][CH 3 CHOHCOO], [Amim][CH 3 The chemical shift of the 2-position hydrogen on the CHOHCOO]imidazole ring δ 2 They are 9.794 ppm and 9.781 ppm respectively. Will δ 2 Substitute into formula 1 to get the two kinds of ionic liquid b The parameter values are 0.964 and 0.945 respectively. [C 4 mim][CH 3 The NMR spectrum of CHOHCOO] as Figure 8 As shown, the NMR spectrum of [Amim][CH3CHOHCOO] is as Figure 9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com