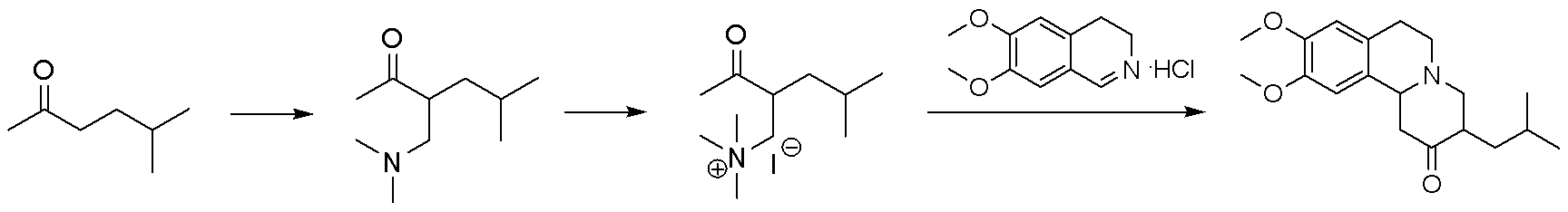
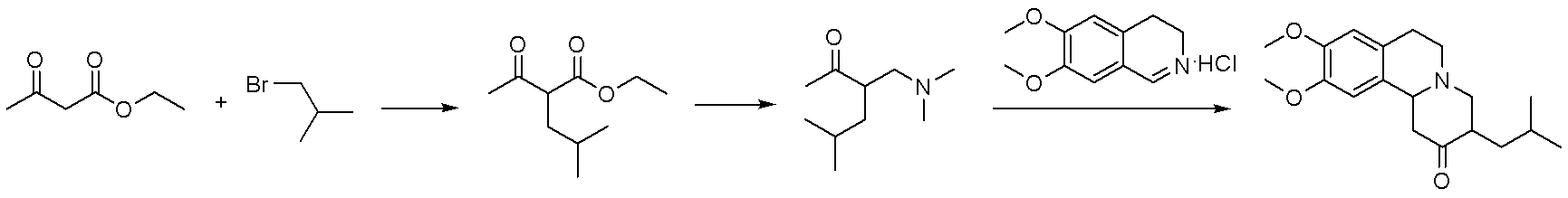
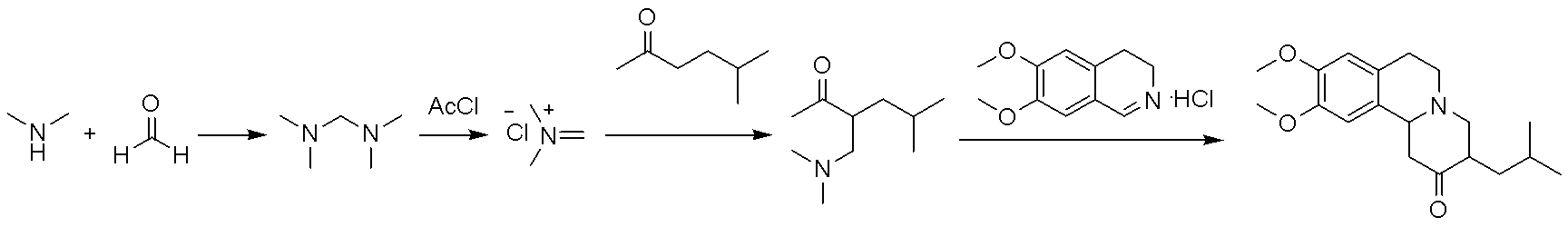
Method for synthesizing tetrabenazine
A technology of tetrabenazine and a synthesis method, which is applied in the field of medicinal chemical synthesis, can solve the problems of high cost of recovery solvent, high cost of raw materials, difficult filtration, etc., achieves good industrial application prospects, improves regional selectivity, and saves cost of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] A kind of synthetic method of tetrabenazine of the present embodiment, comprises the following steps:
[0025] (1) Synthesis of Tetramethylmethylenediamine
[0026] In a 1 L three-necked flask, add 162 g of formaldehyde aqueous solution, and after cooling to 0-5 °C, slowly add 545 g of dimethylamine aqueous solution dropwise. Naturally warm up to room temperature (28°C), and stir for 8 hours. After the reaction solution was cooled to 0-5 °C, 250 g of potassium hydroxide solid was slowly added, and the organic phase was separated and dried with 50 g of sodium hydroxide. After filtration, the organic phase was distilled at atmospheric pressure to obtain 172 g of tetramethylmethylenediamine, with a yield of 84% and a purity of 99% by HPLC.
[0027] (2) Synthesis of 3-[(dimethylamino)methyl]-5-methyl-2-hexanone
[0028] In a 1 L three-necked flask, add 240 ml of N,N-dimethylformamide and 122 g of tetramethylmethylenediamine, after cooling to 0-5 °C, add 94 g of acetyl ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com