Synthetic method of photoinitiator dual (2,4,6-trimethylbenzene formyl group) phenyl phosphine oxide
A trimethylbenzoyl and photoinitiator technology, applied in the chemical industry, can solve problems such as unsafe yields, and achieve the effects of low overall cost, fire resistance, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
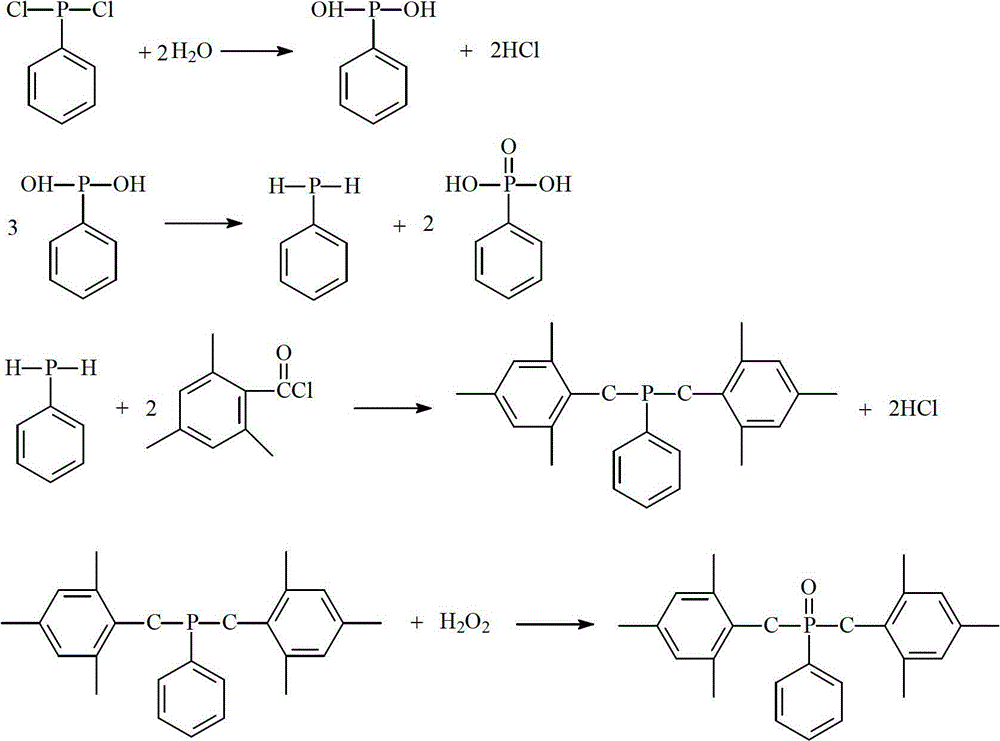
[0021] The first step: add 17.9 grams of phenylphosphine dichloride and 100 mL of toluene in a 250 mL reactor as a solvent, feed nitrogen to remove oxygen, and add 4.0 grams of pure water dropwise under nitrogen protection to control the temperature within 0 ° C. After adding, 0-10 ° C for 2 hours to generate phenylphosphine and by-product phenylphosphonic acid.
[0022] The second step: filter, the filter cake is washed with an appropriate amount of toluene, and the obtained solid is the by-product phenylphosphonic acid; a small amount of anhydrous calcium chloride is added to the filtrate, and the calcium chloride is removed by filtration, and the filtrate is transferred to the reactor, and the temperature is controlled at 60 12.2 grams of 2,4,6-trimethylbenzoyl chloride was added dropwise at °C, and after the addition was completed, it was incubated at 60 °C for 2 hours. After adjusting the reaction solution to neutrality with 10% sodium hydroxide, the organic layer was sepa...
Embodiment 2
[0027] Step 1: Add 17.9 grams of phenylphosphine dichloride and 100 mL of chloroform into a 250 mL reactor as a solvent, feed nitrogen to remove oxygen, control the temperature within 0°C under the protection of nitrogen, add 3.8 grams of pure water dropwise, after adding Insulated at 0-10°C for 2 hours to generate phenylphosphine and by-product phenylphosphonic acid.
[0028] The second step: filtering, the filter cake is washed with an appropriate amount of chloroform, and the obtained solid is the by-product phenylphosphonic acid. Add a small amount of anhydrous calcium chloride in the filtrate, filter and remove calcium chloride, transfer the filtrate in the reactor, control temperature 60 DEG C and drop 12.2 grams of 2,4,6-trimethylbenzoyl chloride, after adding, add Keep warm at 60°C for 2 hours, adjust the reaction solution to neutral with 10% sodium hydroxide after the heat preservation, and separate the organic layer to obtain the intermediate product bis(2,4,6-trimet...
Embodiment 3
[0033] Step 1: Add 17.9 grams of phenylphosphine dichloride to a 250 mL reactor, then add 100 mL as a solvent, pass in nitrogen gas to remove oxygen, control the temperature within 0°C under nitrogen protection, add 3.8 grams of pure water dropwise, after adding Incubate at 80°C for 2 hours.
[0034] The second step: filtering, the filter cake is washed with an appropriate amount of chloroform, and the obtained solid is the by-product phenylphosphonic acid. Add a small amount of anhydrous calcium chloride in the filtrate, filter and remove calcium chloride, transfer the filtrate in the reactor, control temperature 60 DEG C and drop 12.2 grams of 2,4,6-trimethylbenzoyl chloride, after adding, add Keep warm at 60°C for 2 hours, adjust the reaction solution to neutral with 10% sodium hydroxide after the heat preservation, and separate the organic layer to obtain the intermediate product bis(2,4,6-trimethylbenzoyl)phenylphosphine.
[0035] Step 3: Add 14.4 grams of 35% hydrogen p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com