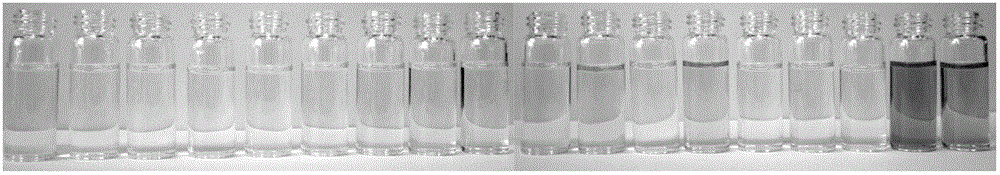Application of rhodamine B thio-bishydrazide derivative as Hg<2+> fluorescent probe
A technology of thiobishydrazide and fluorescent probe, which is applied in the application field of rhodamine B thiobishydrazide derivatives as Hg2+ fluorescent probe, to achieve non-destructive and rapid, extensive social value and economic The effect of efficiency, high selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Using rhodamine B thiobishydrazide derivatives with Hg 2+ Reaction to produce fluorescent rhodamine B-1,3,4-oxadiazole derivatives
[0026] The rhodamine B thiobishydrazide derivative (I) can be prepared according to the method introduced in the applicant's previously applied patent [application number: 201210204864.6].
[0027] Add 50 mL of dichloromethane solution containing 650 mg (1.0 mmol) of thiobishydrazide (I) to 30 mL of 543 mg (2.0 mmol) of HgCl 2 67 mg (0.2 mmol) of tetrabutylammonium bromide was then added to the above mixed solution, and the reaction solution was heated to reflux under stirring, and the reaction was continued for 1.5 hours, and then cooled down. The reaction solution was diluted with 50mL of dichloromethane, allowed to stand for layers, separated, the organic phase was washed with water, dried with anhydrous magnesium sulfate, filtered with suction, and the filtrate was concentrated to obtain 445 mg of a purple-black solid, which was the p...
Embodiment 2
[0035] Rhodamine B Thiobishydrazide Derivatives as Hg 2+ Validation of Fluorescent Probes
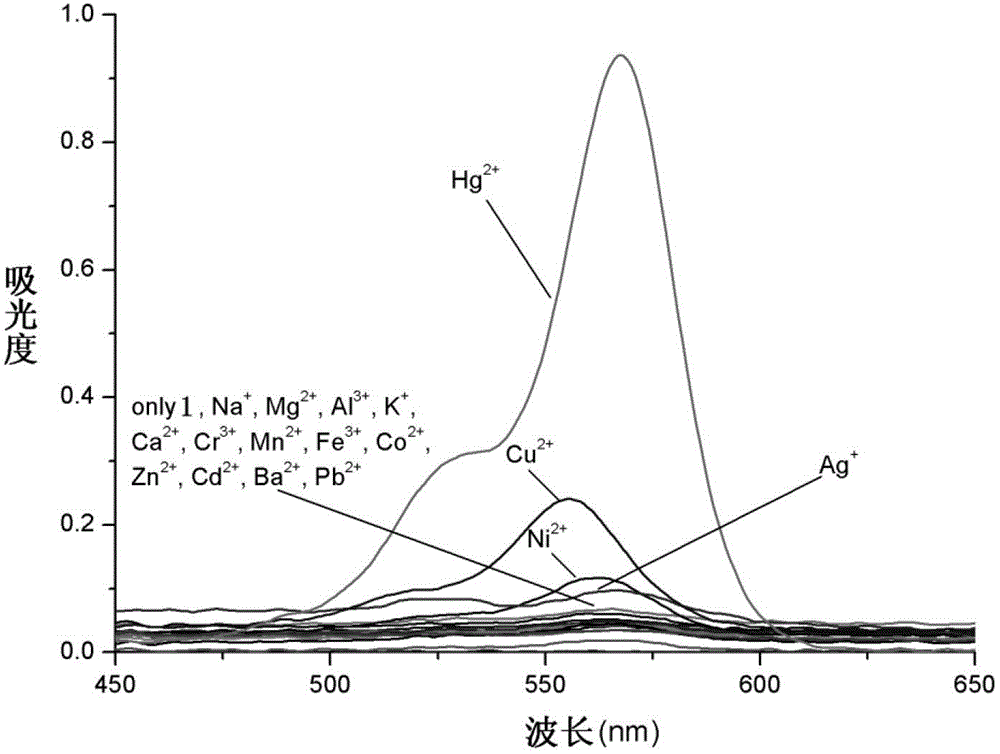
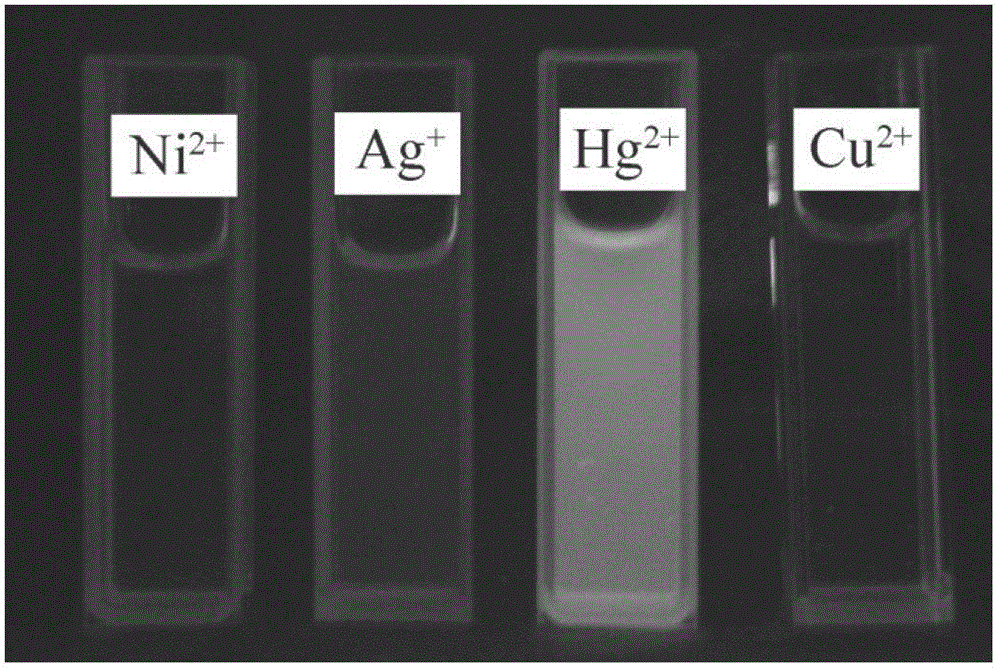
[0036] Add 10 mL of DMF / HEPES buffer solution (20 mM HEPES, pH=7.2, 2:3, volume ratio) containing 10 mL of the prepared rhodamine B thiobishydrazide derivative (1) with a micro-syringe. Equivalent Na + ,Mg 2+ ,Al 3+ , K + , Ca 2+ ,Cr 3+ ,Mn 2+ ,Fe 3+ ,Co 2+ , Ni 2+ ,Zn 2+ , Ag + ,Cd 2+ , Ba 2+ ,Pb 2+ ,Hg 2+ and Cu 2+ The ionic water solution was tested by UV-Vis spectrophotometry and fluorescence spectrophotometry respectively.
[0037] The results showed that the effect of rhodamine B thiobishydrazide derivative (I) on Hg 2+ It has very good selectivity, and the contrast before and after adding mercury ions shows that the fluorescence is enhanced by 23 times, and has a strong fluorescence enhancement effect. (See Figure 4 )
Embodiment 3
[0039] Rhodamine B Thiobishydrazide Derivatives as Hg 2+ Hg in serum 2+ Fluorescence Imaging Test
[0040] with HgCl 2 Feed the experimental mice with different doses for 1 or 2 days, then draw blood and centrifuge to get the serum, and use the mercury ion probe rhodamine B thiobishydrazide derivatives within 5 minutes at room temperature to detect the Hg in the serum. 2+ Perform detection and fluorescence imaging.
[0041] see results Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com